Autoimmune Polyglandular Syndrome Type 1 Market Synopsis
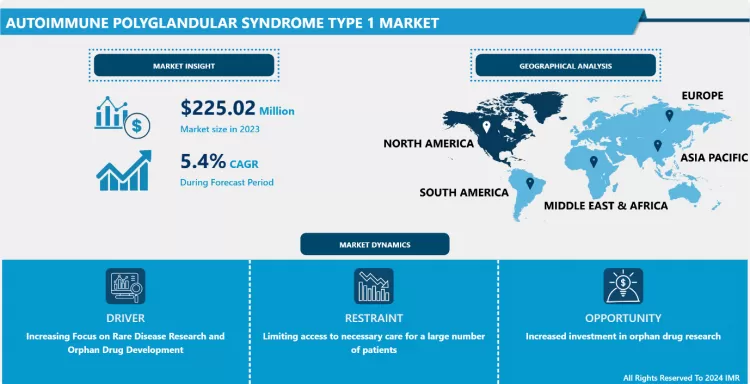
Autoimmune Polyglandular Syndrome Type 1 Market Size Was Valued at USD 225.02 Million in 2023 and is Projected to Reach USD 342.31 Million by 2032, Growing at a CAGR of 5.40% From 2024-2032.
The Autoimmune Polyglandular Syndrome Type 1 (APS-1) market is a significantly smaller and fledging submarket under the umbrella of autoimmune disease market due to its multi-system disease characterised by adrenal insufficiency, chronic mucocutaneous candidiasis, hypoparathyroidism and other autoimmune manifestations. APS-1 is a result of mutated genes in the AIRE gene, with available treatments only being partially curative, such as hormone replacement therapy, antifungal medicine, and immunosuppressive agents. Due to small affected population, underdiagnosis, along with general unavailability of any APS-1 targeted treatments, most patients use medications that are not approved for their condition.
Still, there is swift industry attention from pharmaceutical companies, academic research, and biotechnology firms in gene therapy, biologic, and precision medicine sectors, due to rising understanding of rare diseases and the availability of financial incentives for producing orphan drugs. However, the market for APS-1 could still grow as well as genetics research, diagnostics and government funding for new treatment procedures progress and expand throughout the world. Furthermore, more patient support organizations and the working partnerships between research personnel and drug manufacturers are anticipated to provide solutions to these neglected needs pertaining to this complicated and chronic disease.
- There are AP-1 patients worldwide, but the market is emerging because more attention is being paid to rare autoimmune diseases and the possibility of creating targeted drugs. Hence, APS-1 is genetic disorder due to the mutation in the AIRE gene and patient may have autoimmune adrenal insufficiency, persistent mucocutaneous candidiasis and hypoparathyroidism. To the present, definitive therapy isn’t available; however,, treatment aimed at palliation includes hormone replacement, use of antifungal drugs, and immunosuppressive medications. The market is constricted by the sparseness of the APS-1, reducing the potential patient base, and the current lack of approved targeted treatments for APS-1. It is not just that it opens up new opportunities to improve satisfaction thereby generating more revenue, but it also poses a problem when pharmaceutical companies seek to find a solution to unmet needs of the patients.
- Nevertheless, the potential exists for the APS-1 market growth, especially in view of the developments in genetic therapies, biologics, and precision medicine. While assigning orphan drug status to certain rare diseases like APS-1, governments provide encouragements in the form of tax credit, longer patent protection, and funding opportunity, which could definitely tempt more capital investment in development of APS-1 specific treatments. Secondly, advances in knowledge or diagnostic techniques will also mean enhanced early identification and treatment, enlargement of the patient base and uptake of more specific treatments. Further development in gene therapy and immunomodulation might have implications for APS-1 market creating new treatments that might define clinical and commercial relevance of this relatively rare but effective condition.

Autoimmune Polyglandular Syndrome Type 1 Market Trend Analysis
Increasing Focus on Rare Disease Research and Orphan Drug Development
- The growing emphasis on rare illness research and orphan medication development is helping the market for Autoimmune Polyglandular Syndrome Type 1 (APS-1). Pharmaceutical companies have invested in creating medicines for uncommon diseases like APS-1 because to regulatory incentives offered by orphan drug laws, such as tax breaks, market exclusivity, and expedited clearance procedures. Drug developers find the market more appealing as a result of these incentives, which reduce the financial risks associated with creating treatments for limited patient populations. More financing for APS-1 has also resulted from increased government and private sector investment in rare disease research, which supports the creation of biologics, genetic-based medicines, and other targeted treatments.
- As with APS-1 which is caused by mutations in the AIRE gene, progress in genomic medicine and genetic research is also one of the reasons for this trend.. Thus, although new genes editing technologies such as CRISPR provide the potential for fixing or greatly altering the condition, better diagnostic tools like next-generation sequencing (NGS) are enabling a diagnosis at an earlier stage. We see that this focus on rare diseases is extending innovation even further because academic institutions and pharmaceutical corporations are collaborating to develop novel approaches to treatment. Thus, there are assumptions that the increasing number of approved orphan medications and the increasing share of investment in rare disease research will drive the growth of the APS-1 market, helping people with this challenging autoimmune disease find new ways to treat.
Increased investment in orphan drug research
- There is a huge scope of development and commercialization being created by the increasing focus on orphan drug research in Autoimmune Polyglandular Syndrome Type 1 (APS-1) market.. Since APS-1, is a genetic autoimmune disease of low prevalence, the drug developed for its treatment is likely to be an orphan medication, something that has enormous regulatory advantages such as exclusive market for five years, tax credits and relatively cheap clinical trials. That increases the likelihood of investment in the development of specialized therapeutics by appealing to biotechnology and pharmaceutical companies through offering incentives to those which cause APS-1 to become a more attractive target. These regulatory supports can expedite the development of new therapies for APS-1 patients through covering the financial burdens of a therapy development company for a small number of patients.
- Technological advancement of precision medicine as well as biotechnology is also adding to the rise in the funding for orphan medication development. As funding for rare autoimmune diseases increases, more attention has been given to the development of gene treatments, biologics and immune-modulating medications that may address the cause of APS-1 which includes mutation on the AIRE gene.Also, the time and efficiency with which diagnosis is being done is improving due a range of next-generation sequencing technologies and genetic diagnostics that have potential to bring about early diagnosis with better prognosis for the patient. APS-1 is being increasingly recognized as a promising area for new agents, the amount of disease-specific collaboration between public and private entities offering a glimmer of a better future for rare disease patients and research.
Autoimmune Polyglandular Syndrome Type 1 Market Segment Analysis:
Autoimmune Polyglandular Syndrome Type 1 Market is segmented based on type, application and end user.
By Type, Serum Autoimmune Screen segment is expected to dominate the market during the forecast period
- The Serum Autoimmune Screen segment is expected to lead the Autoimmune Polyglandular Syndrome Type 1 (APS-1) during the forecast period.. Serum autoimmune tests are especially necessary for identifying the definite autoantibodies associated with APS-1, which is a genetic disorder that is characterized by immune mediated destruction of several endocrine organs. These tests help diagnose autoimmune disease symptoms and include chronic mucocutaneous candidiasis, hypoparathyroidism, and adrenal insufficiency which are features of APS-1. The requirement of serum autoimmune screening should be expected due to the fact that it is critical in managing the disorder as early as possible and if not detected uninformed complications can occur.; The serum autoimmune screening plays a critical role in diagnosis in intensive care units.• Moreover there is a projected growth of the serum autoimmune screen market has also prompted by advancement in diagnosis technologies and increased knowledge of rare autoimmune disorders.hroughout the forecast period. Serum autoimmune screenings are crucial for determining the precise autoantibodies linked to APS-1, a rare hereditary condition involving autoimmune reactions that target several endocrine glands. These tests aid in the identification of autoimmune disease signs, such as chronic mucocutaneous candidiasis, hypoparathyroidism, and adrenal insufficiency—all of which are characteristics of APS-1.
- The need for serum autoimmune screening is anticipated to increase since early detection is essential for improved care and avoiding serious consequences, making it a vital diagnostic tool in clinical settings.Additionally, the expansion of the serum autoimmune screen market is anticipated to be driven by improvements in diagnostic technology and heightened awareness of uncommon autoimmune illnesses. As more results of genetic and autoimmune testing become available, the relevant medical personnel will be in a better positioned to diagnose APS-1 early enough with a view of containing the impact through early and appropriate therapy. As serum autoimmune screenings, which are the standard identification procedure for APS-1, this market segment anticipates to hold a high market share, especially with the best diagnostics tool innovation being more accurate and effective as this complex, multi-system disease continues to improve its identification process.
By Application, Hormone Replacement Therapy (HRT) segment expected to held the largest share
- Hormone Replacement Therapy (HRT) segment is anticipated to dominate the market share of Autoimmune Polyglandular Syndrome Type 1 (APS-1) for the span of the forecast period owing to its utility in managing ailment.. APS-1 commonly manifests as primary adrenal insufficiency, hypoparathyroidism, and chronic mucocutaneous candidiasis, diseases for which patients must take hormones throughout their lives to minimize symptoms. APS-1 patients may require corticosteroids for adrenal failure, calcium and Vitamin D, and other hormones when patients have low hormonal levels. Since these therapies are essential in the regulation of normal metabolic processes and to alleviate life threatening complications hormone replacement is the primary treatment in APS-1 making the market dominated by this segment.• Furthermore, currently there is no cure for APS-1 thus HRT is a lifelong therapeutic mainstay in APS-1 patients.Syndrome Type 1 (APS-1) market during the forecast period, driven by its central role in managing the disease. APS-1 typically presents with adrenal insufficiency, hypoparathyroidism, and chronic mucocutaneous candidiasis, conditions that require lifelong hormone replacement for effective symptom management. Patients with APS-1 often need corticosteroids to address adrenal insufficiency, calcium and vitamin D supplements to manage hypoparathyroidism, and other hormones to replace deficient levels. Since these therapies are critical for maintaining normal metabolic functions and preventing life-threatening complications, hormone replacement is the primary treatment approach for APS-1 patients, driving its dominance in the market.
- Moreover, the lack of a cure for APS-1 means that HRT remains a cornerstone of treatment throughout a patient’s life. To this date, there are no disease-modifying medications, so hormonal replacement therapies are crucial for addressing the numerous manifestations associated with the disease, as well as preventing life-threatening consequences such as Addisonian crisis or tetany. As the disease prevalence among APS-1 patients increases because of enhanced diagnostic capabilities and enhanced patient awareness, HRT will remain the largest application segment in the market. Moreover, new findings regarding APS-1 also provide more focused and effective management to patients, thus the development of hormonal therapy will enhance the advancement of the market for research studies on APS-1.
Autoimmune Polyglandular Syndrome Type 1 Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
- Because of the region's sophisticated healthcare system, high level of awareness of rare diseases, and robust support for the development of orphan drugs, North America is anticipated to lead the Autoimmune Polyglandular Syndrome Type 1 (APS-1) market throughout the forecast period. With many pharmaceutical and biotech businesses concentrating on rare diseases and taking advantage of governmental incentives like orphan medication designations, the United States boasts a thriving biopharmaceutical industry. The creation of specialized treatments for APS-1 is facilitated by these incentives, which include tax benefits, expedited approval procedures, and market exclusivity. Furthermore, APS-1 and other uncommon diseases can be better-managed thanks to the widespread availability of early diagnosis and comprehensive healthcare services, which enhances patient outcomes and quality of life. Research on rare diseases continues to be funded thanks to the U.S. Food and Drug Administration (FDA) and other regulatory bodies that help with clinical trials and the approval of new medicines.
- Additionally, there is a strong patient advocacy network in North America that has played a significant role in promoting the development of focused medicines and increasing public awareness of uncommon illnesses like APS-1. In the United States and Canada, organizations and patient groups are essential in advancing financing for research, better diagnostic techniques, and greater patient access to care. Greater patient identification through enhanced genetic testing and diagnostic technologies thus improves the North American market by enabling earlier interventions and more individualized treatment plans. The main treatment modalities for APS-1, hormone replacement therapy and immunomodulatory medications, are also extensively accessible in these areas, which propels the market's expansion. North America is ideally positioned to dominate the APS-1 market throughout the course of the projection period because to a combination of robust healthcare systems, research capabilities, and an increasing emphasis on rare diseases.

Active Key Players in the Autoimmune Polyglandular Syndrome Type 1 Market
- AbbVie Inc. (United States)
- Alexion Pharmaceuticals, Inc. (United States)
- Amgen Inc. (United States)
- Astellas Pharma Inc. (Japan)
- Biomarin Pharmaceutical Inc. (United States)
- Bristol-Myers Squibb Company (United States)
- Eli Lilly and Co. (United States)
- Ferring Pharmaceuticals (Switzerland)
- GlaxoSmithKline PLC (United Kingdom)
- Merck & Co., Inc. (United States)
- Novartis International AG (Switzerland)
- Pfizer Inc. (United States)
- Regeneron Pharmaceuticals, Inc. (United States)
- Sandoz (a division of Novartis) (Switzerland)
- Sanofi Genzyme (France)
- Takeda Pharmaceutical Company (Japan)
- Teva Pharmaceutical Industries Ltd. (Israel)
-
Global Autoimmune Polyglandular Syndrome Type 1 Market
Base Year:
2023
Forecast Period:
2024-2032
Historical Data:
2017 to 2023
Market Size in 2023:
USD 225.02 Billion
Forecast Period 2024-32 CAGR:
5.40%
Market Size in 2032:
USD 342.31 Billion
Segments Covered:
By Type
- Serum Autoimmune Screen
- End-organ Function Tests
- Blood Tests
By Application
- Hormone Replacement Therapy (HRT)
- Medication
By End User
- Hospitals
- Specialty Clinics
- Homecare
By Region
- North America (U.S., Canada, Mexico)
- Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
- Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
- Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New-Zealand, Rest of APAC)
- Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
- South America (Brazil, Argentina, Rest of SA)
Key Market Drivers:
- The increasing emphasis on rare disease research and orphan drug development, backed by government incentives such as tax credits, market exclusivity, and accelerated approval processes, is driving innovation in therapies for APS-1.
Key Market Restraints:
- The cost of diagnostic tests, hormone replacement therapies, and advanced treatments for APS-1 can be prohibitive, especially in low-resource settings, limiting access to necessary care for a large number of patients.
Key Opportunities:
- The increasing availability and adoption of next-generation sequencing (NGS) and advanced serum autoimmune screening will drive earlier and more accurate diagnoses, improving treatment efficacy and patient outcomes
Companies Covered in the report:
- AbbVie Inc. (United States), Alexion Pharmaceuticals, Inc. (United States), Amgen Inc. (United States), Astellas Pharma Inc. (Japan), Biomarin Pharmaceutical Inc. (United States), Bristol-Myers Squibb Company (United States), Eli Lilly and Co. (United States), Ferring Pharmaceuticals (Switzerland). and Other Major Players.
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Autoimmune Polyglandular Syndrome Type 1 Market by By Type (2018-2032)
4.1 Autoimmune Polyglandular Syndrome Type 1 Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Serum Autoimmune Screen
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 End-organ Function Tests
4.5 Blood Tests
Chapter 5: Autoimmune Polyglandular Syndrome Type 1 Market by By Application (2018-2032)
5.1 Autoimmune Polyglandular Syndrome Type 1 Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Hormone Replacement Therapy (HRT)
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Medication
Chapter 6: Autoimmune Polyglandular Syndrome Type 1 Market by By End User (2018-2032)
6.1 Autoimmune Polyglandular Syndrome Type 1 Market Snapshot and Growth Engine
6.2 Market Overview
6.3 Hospitals
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
6.3.3 Key Market Trends, Growth Factors, and Opportunities
6.3.4 Geographic Segmentation Analysis
6.4 Specialty Clinics
6.5 Homecare
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 Autoimmune Polyglandular Syndrome Type 1 Market Share by Manufacturer (2024)
7.1.3 Industry BCG Matrix
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 ABBVIE INC. (UNITED STATES)
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Key Strategic Moves and Recent Developments
7.2.10 SWOT Analysis
7.3 ALEXION PHARMACEUTICALS INC. (UNITED STATES)
7.4 AMGEN INC. (UNITED STATES)
7.5 ASTELLAS PHARMA INC. (JAPAN)
7.6 BIOMARIN PHARMACEUTICAL INC. (UNITED STATES)
7.7 BRISTOL-MYERS SQUIBB COMPANY (UNITED STATES)
7.8 ELI LILLY AND CO. (UNITED STATES)
7.9 FERRING PHARMACEUTICALS (SWITZERLAND)
7.10 GLAXOSMITHKLINE PLC (UNITED KINGDOM)
7.11 MERCK & COINC. (UNITED STATES)
7.12 NOVARTIS INTERNATIONAL AG (SWITZERLAND)
7.13 PFIZER INC. (UNITED STATES)
7.14 REGENERON PHARMACEUTICALS INC. (UNITED STATES)
7.15 SANDOZ (A DIVISION OF NOVARTIS) (SWITZERLAND)
7.16 SANOFI GENZYME (FRANCE)
7.17 TAKEDA PHARMACEUTICAL COMPANY (JAPAN)
7.18 TEVA PHARMACEUTICAL INDUSTRIES LTD. (ISRAEL)
7.19 UCB S.A. (BELGIUM) OTHER KEY PLAYERS
7.20
Chapter 8: Global Autoimmune Polyglandular Syndrome Type 1 Market By Region
8.1 Overview
8.2. North America Autoimmune Polyglandular Syndrome Type 1 Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecasted Market Size By By Type
8.2.4.1 Serum Autoimmune Screen
8.2.4.2 End-organ Function Tests
8.2.4.3 Blood Tests
8.2.5 Historic and Forecasted Market Size By By Application
8.2.5.1 Hormone Replacement Therapy (HRT)
8.2.5.2 Medication
8.2.6 Historic and Forecasted Market Size By By End User
8.2.6.1 Hospitals
8.2.6.2 Specialty Clinics
8.2.6.3 Homecare
8.2.7 Historic and Forecast Market Size by Country
8.2.7.1 US
8.2.7.2 Canada
8.2.7.3 Mexico
8.3. Eastern Europe Autoimmune Polyglandular Syndrome Type 1 Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecasted Market Size By By Type
8.3.4.1 Serum Autoimmune Screen
8.3.4.2 End-organ Function Tests
8.3.4.3 Blood Tests
8.3.5 Historic and Forecasted Market Size By By Application
8.3.5.1 Hormone Replacement Therapy (HRT)
8.3.5.2 Medication
8.3.6 Historic and Forecasted Market Size By By End User
8.3.6.1 Hospitals
8.3.6.2 Specialty Clinics
8.3.6.3 Homecare
8.3.7 Historic and Forecast Market Size by Country
8.3.7.1 Russia
8.3.7.2 Bulgaria
8.3.7.3 The Czech Republic
8.3.7.4 Hungary
8.3.7.5 Poland
8.3.7.6 Romania
8.3.7.7 Rest of Eastern Europe
8.4. Western Europe Autoimmune Polyglandular Syndrome Type 1 Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecasted Market Size By By Type
8.4.4.1 Serum Autoimmune Screen
8.4.4.2 End-organ Function Tests
8.4.4.3 Blood Tests
8.4.5 Historic and Forecasted Market Size By By Application
8.4.5.1 Hormone Replacement Therapy (HRT)
8.4.5.2 Medication
8.4.6 Historic and Forecasted Market Size By By End User
8.4.6.1 Hospitals
8.4.6.2 Specialty Clinics
8.4.6.3 Homecare
8.4.7 Historic and Forecast Market Size by Country
8.4.7.1 Germany
8.4.7.2 UK
8.4.7.3 France
8.4.7.4 The Netherlands
8.4.7.5 Italy
8.4.7.6 Spain
8.4.7.7 Rest of Western Europe
8.5. Asia Pacific Autoimmune Polyglandular Syndrome Type 1 Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecasted Market Size By By Type
8.5.4.1 Serum Autoimmune Screen
8.5.4.2 End-organ Function Tests
8.5.4.3 Blood Tests
8.5.5 Historic and Forecasted Market Size By By Application
8.5.5.1 Hormone Replacement Therapy (HRT)
8.5.5.2 Medication
8.5.6 Historic and Forecasted Market Size By By End User
8.5.6.1 Hospitals
8.5.6.2 Specialty Clinics
8.5.6.3 Homecare
8.5.7 Historic and Forecast Market Size by Country
8.5.7.1 China
8.5.7.2 India
8.5.7.3 Japan
8.5.7.4 South Korea
8.5.7.5 Malaysia
8.5.7.6 Thailand
8.5.7.7 Vietnam
8.5.7.8 The Philippines
8.5.7.9 Australia
8.5.7.10 New Zealand
8.5.7.11 Rest of APAC
8.6. Middle East & Africa Autoimmune Polyglandular Syndrome Type 1 Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecasted Market Size By By Type
8.6.4.1 Serum Autoimmune Screen
8.6.4.2 End-organ Function Tests
8.6.4.3 Blood Tests
8.6.5 Historic and Forecasted Market Size By By Application
8.6.5.1 Hormone Replacement Therapy (HRT)
8.6.5.2 Medication
8.6.6 Historic and Forecasted Market Size By By End User
8.6.6.1 Hospitals
8.6.6.2 Specialty Clinics
8.6.6.3 Homecare
8.6.7 Historic and Forecast Market Size by Country
8.6.7.1 Turkiye
8.6.7.2 Bahrain
8.6.7.3 Kuwait
8.6.7.4 Saudi Arabia
8.6.7.5 Qatar
8.6.7.6 UAE
8.6.7.7 Israel
8.6.7.8 South Africa
8.7. South America Autoimmune Polyglandular Syndrome Type 1 Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecasted Market Size By By Type
8.7.4.1 Serum Autoimmune Screen
8.7.4.2 End-organ Function Tests
8.7.4.3 Blood Tests
8.7.5 Historic and Forecasted Market Size By By Application
8.7.5.1 Hormone Replacement Therapy (HRT)
8.7.5.2 Medication
8.7.6 Historic and Forecasted Market Size By By End User
8.7.6.1 Hospitals
8.7.6.2 Specialty Clinics
8.7.6.3 Homecare
8.7.7 Historic and Forecast Market Size by Country
8.7.7.1 Brazil
8.7.7.2 Argentina
8.7.7.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
|
Global Autoimmune Polyglandular Syndrome Type 1 Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 225.02 Billion |
|
Forecast Period 2024-32 CAGR: |
5.40% |
Market Size in 2032: |
USD 342.31 Billion |
|
Segments Covered: |
By Type |
|
|
|
By Application |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Frequently Asked Questions :
The forecast period in the Autoimmune Polyglandular Syndrome Type 1 Market research report is 2024-2032.
AbbVie Inc. (United States), Alexion Pharmaceuticals, Inc. (United States), Amgen Inc. (United States), Astellas Pharma Inc. (Japan), Biomarin Pharmaceutical Inc. (United States), Bristol-Myers Squibb Company (United States), Eli Lilly and Co. (United States), Ferring Pharmaceuticals (Switzerland). and Other Major Players.
The Autoimmune Polyglandular Syndrome Type 1 Market is segmented into Type, Application, End User and region. By Type, the market is categorized into Serum Autoimmune Screen, End-organ Function Tests, Blood Tests. By Application, the market is categorized into Medication, Hormone Replacement Therapy, Others). By End User, the market is categorized into Hospitals, Specialty Clinics, Homecare, Others. By region, it is analyzed across North America (U.S.; Canada; Mexico), Eastern Europe (Bulgaria; The Czech Republic; Hungary; Poland; Romania; Rest of Eastern Europe), Western Europe (Germany; UK; France; Netherlands; Italy; Russia; Spain; Rest of Western Europe), Asia-Pacific (China; India; Japan; Southeast Asia, etc.), South America (Brazil; Argentina, etc.), Middle East & Africa (Saudi Arabia; South Africa, etc.).
The Autoimmune Polyglandular Syndrome Type 1 (APS-1) market is a significantly smaller and fledging submarket under the umbrella of autoimmune disease market due to its multi-system disease characterised by adrenal insufficiency, chronic mucocutaneous candidiasis, hypoparathyroidism and other autoimmune manifestations. APS-1 is a result of mutated genes in the AIRE gene, with available treatments only being partially curative, such as hormone replacement therapy, antifungal medicine, and immunosuppressive agents. Due to small affected population, underdiagnosis, along with general unavailability of any APS-1 targeted treatments, most patients use medications that are not approved for their condition. Still, there is swift industry attention from pharmaceutical companies, academic research, and biotechnology firms in gene therapy, biologic, and precision medicine sectors, due to rising understanding of rare diseases and the availability of financial incentives for producing orphan drugs. However, the market for APS-1 could still grow as well as genetics research, diagnostics and government funding for new treatment procedures progress and expand throughout the world. Furthermore, more patient support organizations and the working partnerships between research personnel and drug manufacturers are anticipated to provide solutions to these neglected needs pertaining to this complicated and chronic disease.
Autoimmune Polyglandular Syndrome Type 1 Market Size Was Valued at USD 225.02 Billion in 2023, and is Projected to Reach USD 342.31 Billion by 2032, Growing at a CAGR of 5.40% From 2024-2032.








