CAR T cell Therapy Market Synopsis:
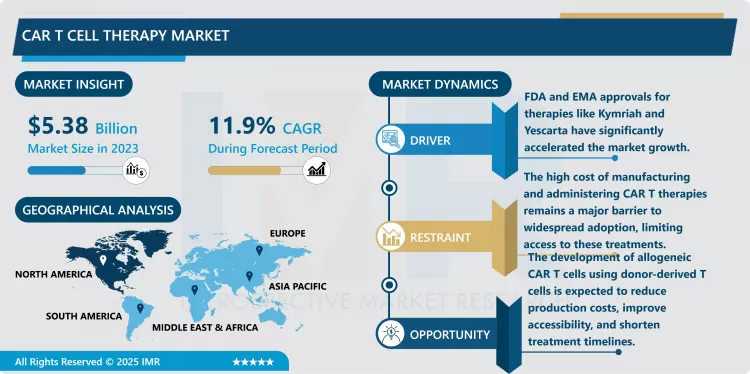
CAR T Cell Therapy Market Size Was Valued at USD 5.38 Billion in 2023, and is Projected to Reach USD 15.23 Billion by 2032, Growing at a CAGR of 11.9% from 2024-2032.
The CAR T Cell Therapy is a still growing category in Oncology and immunotherapy which introduces the Chimeric Antigen Receptor T-cell (CAR T) therapy where, T cells of the patient are genetically modified to target the cancer cells more effectively. Car T cell therapies were first designed for the treatment of diseases like leukaemia, lymphoma and other blood diseases but over the years, they have recorded high success in the treatment of patients who have not responded to common treatments like chemotherapy or radiation. In the last ten years, specialized treatments such as CAR T therapy including Kymriah and Yescarta have gained clearance to be marketed. The therapy involves altering a patient’s T cell in a way that they bind specifically to the cancer cell which acts as a signal to target the tumor. CAR T therapy is currently most effective against blood cancers, the advances in its research are constantly exploring new possibilities with solid tumors including, breast, lung, colon, and others; opening a new market. Drivers of the market include growing cancer prevalence, innovation in gene modification technologies, and growing research in immuno-oncology. However, some barriers to the use of biotechnology in diabetes exist today namely; high costs and the side effects which are associated with the substance as well as scarcity of the substance. Notwithstanding the challenges highlighted above, the market for CAR T cell therapies is projected to rise steadily in the foreseeable future based on technological progresses in tailor-made treatments, pipeline of further development of next generation CAR T, and global availability of the new therapeutic platform. The global market of CAR T cell therapy has seen vigorous progression and has the huge opportunity to alter the course of the cancer treatment and giving hope to various patient populations where currently they do not have effective therapies available for them.
• The CAR T Cell Therapy Market is growing at a tremendous rate mainly because the market is offering a new approach to treat cancer by using the body’s own immune system.. CAR T-cell therapy involves installing in the patients own T-cells a chimeric antigen receptor and due to this ability, kill targeted cancer cells. First used in hematological cancers including leukemia, lymphoma and multiple myeloma, CAR T therapies including Kymriah of Novartis and Yescarta of Gilead have demonstrated high clinical efficacy by offering favourable prognosis for those who have failed to respond to other conventional treatments like chemotherapy or radiotherapy. There is a observed shift away from blood malignancies, with new car ts trials for solid tumors including breast cancer, lung cancer, pancreatic, and many others, which will bring more investment in the field and consequently amplifying the market.• The CAR T Cell Therapy Market is predicted to come up faster due to the following reasons: • The CAR T Cell Therapy market is expected to expand rapidly due to the following reasons:patient's own T cells to express a receptor that targets specific cancer antigens, enabling the immune system to recognize and destroy cancer cells. Initially approved for hematological cancers, such as leukemia, lymphoma, and multiple myeloma, CAR T therapies like Kymriah (Novartis) and Yescarta (Gilead) have shown significant clinical success, offering promising outcomes for patients who have not responded to traditional therapies like chemotherapy or radiation. The market is expanding beyond blood cancers, with a growing number of clinical trials exploring the use of CAR T therapies in solid tumors such as breast cancer, lung cancer, and pancreatic cancer, which has led to increased investment in the sector and further market opportunities.
The CAR T Cell Therapy Market is expected to grow significantly, driven by several key factors, including the rising global incidence of cancer, the increasing adoption of immuno-oncology treatments, and significant advancements in gene-editing technologies. Over time, there will be additional types ofCAR T cell therapies which is receiving regulatory approval as well as expansion of the technology with both the autologous, where patient’s own cells are used in the treatment and allogeneic where donor-derived cells are used. The advance of next-generation CAR T cell therapies for enhanced outcome and lowered toxicity, like dual target CARs, and armored CAR T cells is also expected to fuel the demand. Thus, some problems like critical costly treatment, possible adverse effects, and difficulties in production persist. Nonetheless, the barriers discussed above have not hindered the CAR T cell therapy market for sustained growth and constant involvement across both haematological and more recently, solid tumours, as well as rise in global accessibility to these highly beneficial treatments.

CAR T Cell Therapy Market Trend Analysis:
Expansion Beyond Hematological Malignancies
- Another major shift is the switch of the CAR T Cell Therapy Market from hematological malignancies including leukemia, lymphoma, and multiple myeloma to solid tumors. Although the CAR T cell therapies have proven significant impact to the treatment of blood cancers; however, treating solid tumors is more challenging because of factors such as tumor microenvironment, immune suppression and absence of universal antigens. Nevertheless, there is increasing confidence among physicians and scientists, many clinical trials have been launched to explore the approach of applying CAR T therapies for solid tumor diseases such as breast cancer, lung cancer, pancreatic cancer, and colon cancer and so on. This is likely to expand the market for CAR T therapies even further, as the patient population expands and there is a growing desire for specific treatments for difficult cancers.
- To counter existing issues with solid tumors, new approaches are being developed: bispecific targeting, in which both antigens on CAR T cell receptors are designed to bind targets simultaneously; armored CAR T cells, which are built to enhance T cell activity in the unfavorable environment of the tumor. Third, novel CAR T cell trials are emerging in combination with other IO approaches, such as immune checkpoint inhibitors, which are under investigation in low-phase trials. The effectiveness of the new approaches makes the idea of applying CAR T therapies to different types of cancer more possible, therefore, expansion of CAR T therapies to a wider range of cancer applications is a major driving force for CAR T cell therapy industries in the future years.
Increased Regulatory Approvals Open New Markets
- The report notes that the CAR T Cell Therapy market is preparing for a substantial upswing as expanded regulatory approval leads to extended market entry and fresh opportunities for treatments. The US-FDA and EMA have currently endorsed various CAR T treatments, especially for haematological cancers like ALL, DLBCL and multiple myeloma. CAR T cell therapies remain highly effective in the treatment of blood cancers and the respective regulatory authorities are gradually becoming more permissive to new CAR T cell therapies with broadened indications for other cancers and diseases, including solid tumors such as breast cancer, lung cancer, and pancreatic cancer. This growing acceptance is predicted to open up new markets hence creating a big market for Pharmaceutical companies who are developing CAR T therapies.
- Besides cancer indications, authorities’ approvals are driving the growth of the new-generation CAR T therapies that have better safety profiles, lower complexity for manufacturing and the potential to target pervasive kinds of cancers. CAR T off-the-shelf have also arrived with another massive opportunity to bring affordable and better accessibility of therapy where authorities approved allogeneic cell therapies, derived from donor T cells. As regulatory agencies continue to harmonise approval of CAR T therapies more market access initiatives are expected globally, particularly in developing markets, where cancer rates are increasing and there is increased demand in the advanced cancer treatment. As these new regulations approvals for treatment create new market opportunities for growth and CAR T Cell Therapy Market innovations, the outlook for cancer patients will continue to improve.
CAR T Cell Therapy Market Segment Analysis:
CAR T Cell Therapy Market is Segmented on the basis of Type, Application, End-User, and Region
By Type, Autologous CAR T Cell Therapy segment is expected to dominate the market during the forecast period
- It is in the Autologous CAR T Cell Therapy segment that analysts project the growth of the CAR T Cell Therapy Market throughout the forecast period. Autologous CAR T therapy means that the patient’s T cells taken from the body, then are genetically altered in the laboratory to include a chimeric antigen receptor (CAR) and then are reintroduced into the patient to fight cancer. This kind of therapy is already exhibiting great clinical efficacy, especially in hematopoietic tumor diseases like ALL, non-Hodgkin’s lymphoma, and multiple myeloma; for patients who have failed chemotherapy and radiation therapy.
- The combinantial share dominance of autologous CAR T cell therapies mainly depends on the already approved products such as Kymriah of Novartis and Yescarta from Gilead that are the first-line treatment for blood cancer. These therapies have laid down the framework of how CAR T will be administered and have garnered much acclaim for its potential to cure, specifically, several types of cancer through development of remission. Therefore, based on clinical data supporting the use of autologous CAR T cell therapy where it has shown greatest benefit in hematological cancers this segment will continue to be the largest market share of the CAR T market. Besides, as innovative and efficiency in production and various processes increase, this segment’s market share will have a tendency to grow with more and more hospitals and treatment centres using it across the world.
By Application, Hematological Malignancies segment expected to held the largest share
- The segment covering Hematological Malignancies is expected to dominate the CAR T Cell Therapy Market during the given forecast period. Hematological cancers such as ALL, NHL, CLL and multiple myeloma are the most targeted diseases by CAR T cell therapies to date. This is primarily because of the very promising outcomes of autologous CAR T therapies such as Kymriah and Yescarta in treating these blood cancers when other treatments such as chemotherapy or bone marrow transplants may not be ideal for all patients particularly those with relapsed or refractory disease.
- Anti-cancer immunotherapy CAR T cells which deploy the body’s immune system to find and kill cancer cells have refreshed outcomes in several hematological cancers to deliver long-term survival profiles and even cure possibly untreatable patients. These promising results have paved way to increased use of CAR T therapies in dealing with blood cancers as it constitutes significantly high market share of the CAR T Cell Therapy Market. Further new therapies will enter the regulatory approval and clinical trials for hematological cancers will remain successful, thus segment will be the biggest application area. Besides, the clearance of new CAR T therapies and further development of treatments will also be contributing to the growth of this segment which will help Novartis to remain as the market share holder.
CAR T Cell Therapy Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
- North America is expected to account for the largest share in the CAR T Cell Therapy Market during the forecast period due to factors as follows – well-developed healthcare system, greater health care expenditure, strong research and development, and favorable regulatory approvals. This dominance owes to the United States, whereby the FDA has okayed a few CAR T cell therapies including Kymriah (Novartis) and Yescarta (Gilead), for the handling of hematological malignancies. The approvals received have made these molecules widely accepted for use in several clinics across the country and the setting up of specific treatment centers. Furthermore, increase in the significant proportion of the healthcare industry and high demand for advanced cancer treatment technologies is expected to increase the investment in CAR T cell therapy, augmenting the regional market growth.
- Further, North America including Canada is also participating in the growth of CAR T cell therapy market with continuously enhancing partnerships between biotech companies, academic institutes and healthcare facilities focusing on developing CAR T. In North America, due largely to the increasing incidence of cancer especially hematological malignancies, positive reimbursement policies and environment make North America the largest market for CAR T cell therapies. This is complimented by the leadership in clinical trials, manufacturing capacity and health care system in the region making it even more dominant. North America remains well positioned to maintain its position as a dominant player in the global CAR T market, as approval of molecular therapies continue to expand commercially into more cancer indications and solid tumor targets.
Active Key Players in the CAR T Cell Therapy Market:
- AbbVie Inc. (United States)
- Amgen Inc. (United States)
- Autolus Therapeutics (United Kingdom)
- Bayer AG (Germany)
- Bluebird Bio, Inc. (United States)
- Bristol Myers Squibb Company (United States)
- Celgene Corporation (Acquired by Bristol Myers Squibb) (United States)
- Celyad Oncology (Belgium)
- Fate Therapeutics, Inc. (United States)
- Genmab A/S (Denmark)
- Gilead Sciences, Inc. (United States)
- Juno Therapeutics (A Bristol-Myers Squibb Company) (United States)
- Kite Pharma (A Gilead Company) (United States)
- Medigene AG (Germany)
- Novartis International AG (Switzerland)
- Sorrento Therapeutics, Inc. (United States)
- Takeda Pharmaceutical Company Limited (Japan)
- Vertex Pharmaceuticals Incorporated (United States)
- Other Active Players
|
CAR T Cell Therapy Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 5.38 Billion |
|
Forecast Period 2024-32 CAGR: |
11.9 % |
Market Size in 2032: |
USD 15.23 Billion |
|
Segments Covered: |
By Type |
|
|
|
By Application |
|
||
|
By End-User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: CAR T cell Therapy Market by By Type (2018-2032)
4.1 CAR T cell Therapy Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Autologous CAR T Cell Therapy
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 Allogeneic CAR T Cell Therapy
4.5 Next-Generation CAR T Cells
Chapter 5: CAR T cell Therapy Market by By Application (2018-2032)
5.1 CAR T cell Therapy Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Hematological Malignancies (Blood Cancers)
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Solid Tumors
Chapter 6: CAR T cell Therapy Market by By End-User (2018-2032)
6.1 CAR T cell Therapy Market Snapshot and Growth Engine
6.2 Market Overview
6.3 Hospitals
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
6.3.3 Key Market Trends, Growth Factors, and Opportunities
6.3.4 Geographic Segmentation Analysis
6.4 Specialty Clinics
6.5 Academic and Research Institutions
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 CAR T cell Therapy Market Share by Manufacturer (2024)
7.1.3 Industry BCG Matrix
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 ABBVIE INC. (UNITED STATES)
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Key Strategic Moves and Recent Developments
7.2.10 SWOT Analysis
7.3 AMGEN INC. (UNITED STATES)
7.4 AUTOLUS THERAPEUTICS (UNITED KINGDOM)
7.5 BAYER AG (GERMANY)
7.6 BLUEBIRD BIO INC. (UNITED STATES)
7.7 BRISTOL MYERS SQUIBB COMPANY (UNITED STATES)
7.8 CELGENE CORPORATION (ACQUIRED BY BRISTOL MYERS SQUIBB) (UNITED STATES)
7.9 CELYAD ONCOLOGY (BELGIUM)
7.10 FATE THERAPEUTICS INC. (UNITED STATES)
7.11 GENMAB A/S (DENMARK)
7.12 GILEAD SCIENCES INC. (UNITED STATES)
7.13 JUNO THERAPEUTICS (A BRISTOL-MYERS SQUIBB COMPANY) (UNITED STATES)
7.14 KITE PHARMA (A GILEAD COMPANY) (UNITED STATES)
7.15 MEDIGENE AG (GERMANY)
7.16 NOVARTIS INTERNATIONAL AG (SWITZERLAND)
7.17 SORRENTO THERAPEUTICS INC. (UNITED STATES)
7.18 TAKEDA PHARMACEUTICAL COMPANY LIMITED (JAPAN)
7.19 VERTEX PHARMACEUTICALS INCORPORATED (UNITED STATES)
7.20 OTHER ACTIVE PLAYERS
Chapter 8: Global CAR T cell Therapy Market By Region
8.1 Overview
8.2. North America CAR T cell Therapy Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecasted Market Size By By Type
8.2.4.1 Autologous CAR T Cell Therapy
8.2.4.2 Allogeneic CAR T Cell Therapy
8.2.4.3 Next-Generation CAR T Cells
8.2.5 Historic and Forecasted Market Size By By Application
8.2.5.1 Hematological Malignancies (Blood Cancers)
8.2.5.2 Solid Tumors
8.2.6 Historic and Forecasted Market Size By By End-User
8.2.6.1 Hospitals
8.2.6.2 Specialty Clinics
8.2.6.3 Academic and Research Institutions
8.2.7 Historic and Forecast Market Size by Country
8.2.7.1 US
8.2.7.2 Canada
8.2.7.3 Mexico
8.3. Eastern Europe CAR T cell Therapy Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecasted Market Size By By Type
8.3.4.1 Autologous CAR T Cell Therapy
8.3.4.2 Allogeneic CAR T Cell Therapy
8.3.4.3 Next-Generation CAR T Cells
8.3.5 Historic and Forecasted Market Size By By Application
8.3.5.1 Hematological Malignancies (Blood Cancers)
8.3.5.2 Solid Tumors
8.3.6 Historic and Forecasted Market Size By By End-User
8.3.6.1 Hospitals
8.3.6.2 Specialty Clinics
8.3.6.3 Academic and Research Institutions
8.3.7 Historic and Forecast Market Size by Country
8.3.7.1 Russia
8.3.7.2 Bulgaria
8.3.7.3 The Czech Republic
8.3.7.4 Hungary
8.3.7.5 Poland
8.3.7.6 Romania
8.3.7.7 Rest of Eastern Europe
8.4. Western Europe CAR T cell Therapy Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecasted Market Size By By Type
8.4.4.1 Autologous CAR T Cell Therapy
8.4.4.2 Allogeneic CAR T Cell Therapy
8.4.4.3 Next-Generation CAR T Cells
8.4.5 Historic and Forecasted Market Size By By Application
8.4.5.1 Hematological Malignancies (Blood Cancers)
8.4.5.2 Solid Tumors
8.4.6 Historic and Forecasted Market Size By By End-User
8.4.6.1 Hospitals
8.4.6.2 Specialty Clinics
8.4.6.3 Academic and Research Institutions
8.4.7 Historic and Forecast Market Size by Country
8.4.7.1 Germany
8.4.7.2 UK
8.4.7.3 France
8.4.7.4 The Netherlands
8.4.7.5 Italy
8.4.7.6 Spain
8.4.7.7 Rest of Western Europe
8.5. Asia Pacific CAR T cell Therapy Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecasted Market Size By By Type
8.5.4.1 Autologous CAR T Cell Therapy
8.5.4.2 Allogeneic CAR T Cell Therapy
8.5.4.3 Next-Generation CAR T Cells
8.5.5 Historic and Forecasted Market Size By By Application
8.5.5.1 Hematological Malignancies (Blood Cancers)
8.5.5.2 Solid Tumors
8.5.6 Historic and Forecasted Market Size By By End-User
8.5.6.1 Hospitals
8.5.6.2 Specialty Clinics
8.5.6.3 Academic and Research Institutions
8.5.7 Historic and Forecast Market Size by Country
8.5.7.1 China
8.5.7.2 India
8.5.7.3 Japan
8.5.7.4 South Korea
8.5.7.5 Malaysia
8.5.7.6 Thailand
8.5.7.7 Vietnam
8.5.7.8 The Philippines
8.5.7.9 Australia
8.5.7.10 New Zealand
8.5.7.11 Rest of APAC
8.6. Middle East & Africa CAR T cell Therapy Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecasted Market Size By By Type
8.6.4.1 Autologous CAR T Cell Therapy
8.6.4.2 Allogeneic CAR T Cell Therapy
8.6.4.3 Next-Generation CAR T Cells
8.6.5 Historic and Forecasted Market Size By By Application
8.6.5.1 Hematological Malignancies (Blood Cancers)
8.6.5.2 Solid Tumors
8.6.6 Historic and Forecasted Market Size By By End-User
8.6.6.1 Hospitals
8.6.6.2 Specialty Clinics
8.6.6.3 Academic and Research Institutions
8.6.7 Historic and Forecast Market Size by Country
8.6.7.1 Turkiye
8.6.7.2 Bahrain
8.6.7.3 Kuwait
8.6.7.4 Saudi Arabia
8.6.7.5 Qatar
8.6.7.6 UAE
8.6.7.7 Israel
8.6.7.8 South Africa
8.7. South America CAR T cell Therapy Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecasted Market Size By By Type
8.7.4.1 Autologous CAR T Cell Therapy
8.7.4.2 Allogeneic CAR T Cell Therapy
8.7.4.3 Next-Generation CAR T Cells
8.7.5 Historic and Forecasted Market Size By By Application
8.7.5.1 Hematological Malignancies (Blood Cancers)
8.7.5.2 Solid Tumors
8.7.6 Historic and Forecasted Market Size By By End-User
8.7.6.1 Hospitals
8.7.6.2 Specialty Clinics
8.7.6.3 Academic and Research Institutions
8.7.7 Historic and Forecast Market Size by Country
8.7.7.1 Brazil
8.7.7.2 Argentina
8.7.7.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
|
CAR T Cell Therapy Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 5.38 Billion |
|
Forecast Period 2024-32 CAGR: |
11.9 % |
Market Size in 2032: |
USD 15.23 Billion |
|
Segments Covered: |
By Type |
|
|
|
By Application |
|
||
|
By End-User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Frequently Asked Questions :
The forecast period in the CAR T Cell Therapy Market research report is 2024-2032.
AbbVie Inc. (United States), Amgen Inc. (United States), Autolus Therapeutics (United Kingdom), Bayer AG (Germany), Bluebird Bio, Inc. (United States), Bristol Myers Squibb Company (United States), Celgene Corporation (Acquired by Bristol Myers Squibb) (United States), Celyad Oncology (Belgium), Fate Therapeutics, Inc. (United States), Genmab A/S (Denmark), Gilead Sciences, Inc. (United States), Juno Therapeutics (A Bristol-Myers Squibb Company) (United States), Kite Pharma (A Gilead Company) (United States), Medigene AG (Germany), Novartis International AG (Switzerland), Sorrento Therapeutics, Inc. (United States), Takeda Pharmaceutical Company Limited (Japan), Vertex Pharmaceuticals Incorporated (United States), and Other Active Players.
The CAR T Cell Therapy Market is segmented into Type, Application, End User and region. By Type, the market is categorized into Autologous CAR T Cell Therapy,Allogeneic CAR T Cell Therapy,Next-Generation CAR T Cells. By Application, the market is categorized into Hematological Malignancies), Solid Tumors, Other Application. By End User, the market is categorized into Hospitals, Specialty Clinics, Academic and Research Institutions. By region, it is analyzed across North America (U.S., Canada, Mexico), Eastern Europe (Russia, Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe), Western Europe (Germany, UK, France, The Netherlands, Italy, Spain, Rest of Western Europe), Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New-Zealand, Rest of APAC), Middle East & Africa (Turkiye, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa), South America (Brazil, Argentina, Rest of SA)
The CAR T Cell Therapy is a still growing category in Oncology and immunotherapy which introduces the Chimeric Antigen Receptor T-cell (CAR T) therapy where, T cells of the patient are genetically modified to target the cancer cells more effectively. Car T cell therapies were first designed for the treatment of diseases like leukaemia, lymphoma and other blood diseases but over the years, they have recorded high success in the treatment of patients who have not responded to common treatments like chemotherapy or radiation. In the last ten years, specialized treatments such as CAR T therapy including Kymriah and Yescarta have gained clearance to be marketed. The therapy involves altering a patient’s T cell in a way that they bind specifically to the cancer cell which acts as a signal to target the tumor. CAR T therapy is currently most effective against blood cancers, the advances in its research are constantly exploring new possibilities with solid tumors including, breast, lung, colon, and others; opening a new market. Drivers of the market include growing cancer prevalence, innovation in gene modification technologies, and growing research in immuno-oncology. However, some barriers to the use of biotechnology in diabetes exist today namely; high costs and the side effects which are associated with the substance as well as scarcity of the substance. Notwithstanding the challenges highlighted above, the market for CAR T cell therapies is projected to rise steadily in the foreseeable future based on technological progresses in tailor-made treatments, pipeline of further development of next generation CAR T, and global availability of the new therapeutic platform. The global market of CAR T cell therapy has seen vigorous progression and has the huge opportunity to alter the course of the cancer treatment and giving hope to various patient populations where currently they do not have effective therapies available for them.
CAR T Cell Therapy Market Size Was Valued at USD 5.38 Billion in 2023, and is Projected to Reach USD 15.23 Billion by 2032, Growing at a CAGR of 11.9% from 2024-2032.








