Cell Penetrating Peptide Market Synopsis:
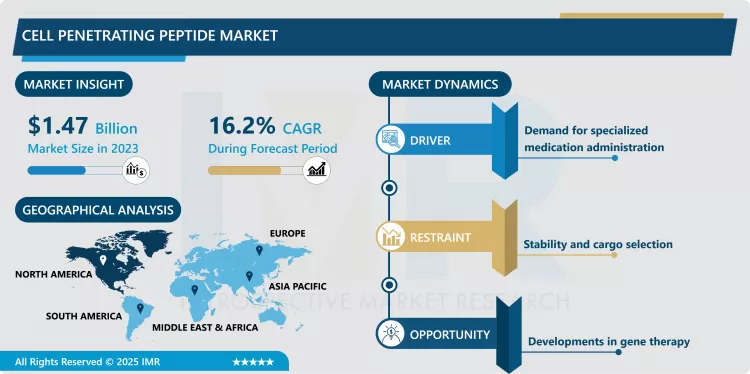
Cell Penetrating Peptide Market Size Was Valued at USD 1.47 Billion in 2023, and is Projected to Reach USD 7.15 Billion by 2032, Growing at a CAGR of 16.2 % From 2024-2032.
The global cell-penetrating peptide (CPP) market is one of the promising segments for the biotechnology and pharmaceutical companies. This is because increasing numbers of individuals require efficient methods for drug delivery, particularly when the drug is not easily transported across cellular membranes by proteins, nucleic acids or small molecules. CPPs are nearly always cationic and usually quite short peptides which facilitate the entry of these medicinal agents into cells. This renders them more available in the body and potent, and with less side effects as compared to their non-nano counterparts. The market is growing due to developments in molecular biology, better understanding of the need for long term perhaps life-long management of diseases, such as cancer or inherited genetic disorders, and an overall increase in investment in biopharmaceuticals. Using CPPs have be found useful in drug delivery, gene therapy, and vaccine development to mention but a few. They are very much wanted by pharmaceutical firms, university research laboratories, and contract research organizations. However, issues such as legal issues, stability, and Bioavailability concerns never cease to pop up and complicate CPP based therapies’ development and marketting. In general, the CPP market is expected to continue to expand as long as new technologies and treatments are produced, and individuals seek new strategies to address diseases.
Cell-penetrating peptides (CPPs) have recently been a focus of the market because the usage of efficient drug delivery systems to transport substances across cell membranes continues to grow.. CPPs are short peptides that enhance the ability of bioactive molecules, including proteins, nucleic acids and small compounds to be transported. This makes them very advantageous within the drug studies as well as gene therapy. Today there are more and more consumers seeking to tailor-made treatments for their conditions, especially chronic diseases such as cancer and hereditary diseases. Due to advances in research and advances in peptide synthesis, there are more CPPs available to the scientific community and tremendous investment in biopharmaceuticals is making it easier to find uses for them. However, the market encounters issues including complex regulatory framework, concerns regarding stability and bioavailability and toxicity it poses, which makes its journey to clinical application even more challenging.By type, there is the possibility of categorizing CPPs into groups.changing quickly because more and more people want effective drug delivery methods that can move medicines across cell membranes. CPPs are short peptides that make it easy for bioactive molecules like proteins, nucleic acids, and small compounds to be taken in. This makes them very useful in drug research and gene therapy. But the market meets problems, such as complicated rules, worries about stability and bioavailability, and possible toxicity issues, which can all make the way to clinical use more difficult.CPPs can be divided into groups based on their type. The most frequently used are cationic peptides as cationic type readily adheres on the negatively charged cell walls. Drug delivery, gene therapy, molecular imaging and vaccine development are only some examples of the applications that pharmaceutical companies, university research institutions and contract research organizations (CROs) are concerned with. Large global manufacturers are already in the market along with the newer biotech ventures and a lot more activities come from universities cooperating with business. With the advancement of the CPP market, people involved in it find a lot of opportunities to look for new ways to treat people with the help of new study and technology.

Cell Penetrating Peptide Market Trend Analysis:
Enhanced Attention to Customized Medicine
- By integrating the perception of personalized or customize medicine with the market of cell penetrating peptides (CPPs), it is seen that there is a significantly move towards a more patient specific approach in the healthcare delivery.. Due to a possibility of delivering medication according to individual patterns, CPPs are well placed to be actively involved in this shift. CPPs enhance therapeutic engagement by enabling the targeted delivery of therapeutic cargoes to particular cells or tissues through a process that avoids interference from circulation and normal bodily processes. For this reason, compared to general drug delivery systems that may have unwanted side effects but high therapeutic efficacy, CPPs are ideal for those designing novel targeted treatments.Further since CPPs can transport diverse therapeutic cargos, their versatility and flexibility make them suitable for delivery of any type of payload. This aspect enables one to develop particular treatment profiles that reflect on each patient’s genotype and the prevailing disease. In the era of genomic medicine and such technologies as CRISPR/Cas9 to modify genes the need for effective vectors to deliver the cell-targeted molecules is rising. The use of CPPs to deliver gene-editing components is becoming more common, meaning that it becomes possible to devise treatment for inherited disease in a more personalized manner. This integration of CPPs with genomic approaches provides strong evidence for the role of CPPs in the further development of personalized medicine.• As the concept of patient-oriented care grows stronger, more and more clinical trials are including CPPs to evaluate how they are useful for targeting therapies for specific patients.hcare toward treatments catered to the specific requirements of each patient. Because of its capacity to enable tailored medication delivery, CPPs are in a unique position to play a significant part in this transition.
- As the focus on customized medicine intensifies, many clinical trials are now incorporating CPPs into their protocols, exploring their effectiveness in delivering personalized therapies. Besides the illustration of the benefits of CPPs in this work, the authors aimed at enhancing the design of these development plans for optimizing clinical results. Further it is shown that a focus is made on patient-centred care on the basis of which, CPPs can assist with enhancing results as well. Notably, there is also a positive shift in the regulatory framework in regards to personalised medicine projects hence creating a conducive market for approval of CPP based therapies All factors considered the CPP industry is predicted to expand since the demand for personalised medication is continually rising. This is because CPPs will be useful in designing conforming treatments relevant to patients and the innovative therapeutic management tool. It weights to the fact that the overall trend in delivering healthcare is towards individualized effective treatments and putting CPPs at the fore front of offering new solutions in medical field.
Meeting the rising demand for personalized medicine
- The market for cell-penetrating peptides (CPPs) is in a unique position to meet the rising need for customized medicine, which places an emphasis on specialized treatment plans that take into account each patient's unique characteristics. CPPs become essential instruments for effectively and efficiently administering targeted therapeutics as healthcare moves toward more customized treatments. Their capacity to make it easier for different therapeutic agents—like proteins, nucleic acids, and tiny molecules—to enter particular cells directly improves treatment precision and enables tailored strategies that benefit patients.CPPs are very useful in gene therapy applications because they make it possible to efficiently distribute genetic material that is customized to each patient's own genetic profile. This skill is crucial for creating focused treatments for cancer and hereditary diseases, which frequently call for individualized approaches. The need for efficient delivery systems like CPPs is anticipated to grow as gene treatments become more widely accepted and regulatory frameworks change to facilitate their approval.
- Furthermore, CPPs are flexible drug delivery platforms because their sequences can be changed to suit certain therapeutic requirements. In a time when customized medicine aims to tailor treatment plans according to unique patient characteristics, such as genetic predispositions and disease states, this adaptability is essential. Healthcare professionals can provide more individualized and efficient therapies that are consistent with precision medicine concepts by using CPP technology into clinical practice.The function of CPPs in personalized medicine is being further enhanced by the continuous developments in peptide engineering and formulation. To increase the efficacy and specificity of CPPs and enable therapy to be customized to meet the various needs of patients, researchers are investigating novel CPP combinations and alterations. CPPs have the potential to significantly alter the field of personalized medicine as long as industry-academia collaborations continue to stimulate innovation.
Cell Penetrating Peptide Market Segment Analysis:
Cell Penetrating Peptide Market is Segmented on the basis of Type, Application End User, and Region
By Type, protein-based cpps segment segment is expected to dominate the market during the forecast period
- The market for protein-based cell-penetrating peptides (CPPs) is dominated by this category because to its versatility, dependability, and biocompatibility. Because these CPPs are made from natural proteins, there is less chance that they would cause unfavorable immunological reactions, which further improves their safety profile. It is crucial for therapeutic uses because, within tissues, it is better to engage human compatibility intrinsically, leading to a more favorable interaction with tissues of the patient. In addition, protein-based CPPs have already been demonstrated to be safe and efficient for various therapeutic payloads including proteins, nucleic acids and other molecular cargoes. They are therefore suitable to be used by scientists and pharmaceutical companies seeking to develop new cure products.
- CPPs based on proteins provide a strong foundation for developing precision treatments. Their capacity to make complicated biologics easier to distribute fits very nicely with the growing need for individualized and focused therapy. In addition to improving the effectiveness of these treatments, this integration with current biopharmaceuticals and medication formulations establishes protein-based CPPs as a crucial element in the changing field of drug development. These peptides' well-established clinical uses in a range of therapeutic domains, including as cancer, genetic disorders, and infectious diseases, further support their market presence.In contrast, the peptide-based CPP segment is expected to develop significantly over the course of the projection period, with a projected compound annual growth rate (CAGR) of 14.7%. This expansion can be mostly attributed to the intrinsic benefits that peptide-based CPPs provide. Compared to alternative delivery methods, peptides have a high degree of biocompatibility, which results in less toxicity and immunogenicity issues. Their sequences can be readily modified to satisfy particular needs, enabling targeted molecular activities such as medication delivery. Because of their adaptability, scientists can alter peptides to increase therapeutic cargo delivery and cellular uptake.
By Application, Gene delivery segment expected to held the largest share
- There is high interest in market for cell-penetrating peptides (CPPs) as the approach to such a form of medication as personalized or custom-made medicine, and therefore the medicine itself is rapidly developing depending on personal necessity of every patient.. Owing to its ability to facilitate personalised medication administration, cpps are well placed to have a large role in this process. CPPs enhance the treatment accuracy since they help in the targeting and direct administration of therapeutic agents such as drugs, proteins and genes into specific cell or tissues. In a nutshell, CPPs are a favourable option for those developing individualised techniques owing to the low impact on other processes while an optimal effect is achieved CPPs are highly flexible and can deliver a vast amount of therapeutic cargo. This feature provides for the provision of treatment that is individualised based on the genetic makeup and current illness of the patient. In particular, as genomic medicine develops in addition to gene editing technologies being able to correct disease causing genes using CRISPR there is a growing need for unique delivery systems. Several types of molecular systems are used to deliver gene-editing components and since gene therapies are available with disease-modifying targets, the use of CPPs can allow for individually tailored medicine for rare genetic diseases. The ability to incorporate CPPs into the development of personalized medicine is further established by its convergence with genomic methodologies.• In recent years, many trials are involved CPPs in their treatment procedures, since the emphasis on customized medication increases.hcare toward treatments catered to the specific requirements of each patient. As a result of their ability to facilitate targeted drug delivery, CPPs have the potential to assume an important role in this process.
- CPPs enhance the value of treatment accuracy by ensuring that therapeutic agents such as drugs, proteins, and genes get to work exactly on some chosen cells or tissues.ilitating the direct delivery of therapeutic substances, including medications, proteins, and genetic materials, into certain cells or tissues. Firstly, and largely due to their high selectivity where side effects are addressed while therapeutic benefits enhanced, CPPs are preferred by those designing targeted treatments Secondly, and consistent with their flexible and adaptable nature, CPPs can transport a plethora of therapeutic cargos. Such feature allow implementing individualized approach while working on treatment options based on the genetic predisposition of the patient and the illness at the moment.
With the rise of genomic medicine and advanced gene editing technologies like CRISPR, the demand for efficient delivery systems that can specifically target cells is increasing. CPPs are increasingly utilized to deliver gene-editing components, making it possible to customize therapies for genetic disorders on a patient-by-patient basis. The relevance of CPPs in the advancement of customized medicine is further cemented by this integration with genomic approaches.
Cell Penetrating Peptide Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
- Due to a number of important factors, North America is expected to continue to dominate the cell-penetrating peptide (CPP) market for the duration of the projected period. The region is home to a thriving pharmaceutical and biotechnology sector that is distinguished by large R&D investments. Large corporations and academic organizations in the US and Canada are actively investigating CPPs for a range of therapeutic uses, such as vaccine production, gene therapy, and cancer treatment. This strong research environment encourages creativity and speeds up the clinical use of CPP technology.Furthermore, the need for cutting-edge treatment solutions is being fueled by the rising incidence of genetic abnormalities and chronic diseases in North America. CPPs are essential to the development of targeted therapeutics because of their acknowledged capacity to improve the delivery of nucleic acids and biologics. Interest in CPPs is anticipated to increase as patients and healthcare professionals look for more potent treatment choices, hence enhancing North America's position as a market leader.
- Favorable regulatory environments that encourage the creation and acceptance of innovative treatments are also helping the area. The commercialization of CPP-based medicines is made possible by regulatory bodies like Health Canada and the U.S. Food and Drug Administration (FDA) being more receptive to novel delivery methods. Furthermore, the region's dedication to developing this discipline is demonstrated by the large number of clinical trials assessing CPPs in diverse therapeutic scenarios.Another important element driving market expansion in North America is cooperation between academia and business. Collaborations and alliances make it easier to share resources and information, which improves research capacities and results in the creation of innovative CPP technologies. Additionally, the region's growing emphasis on personalized medicine complements CPPs' ability to provide customized therapeutics, which propels market growth.
Active Key Players in the Cell Penetrating Peptide Market:
- Amino Labs (USA)
- Apexigen, Inc. (USA)
- Bachem Holding AG (Switzerland)
- BiomX Inc. (Israel)
- CureVac AG (Germany)
- CytomX Therapeutics, Inc. (USA)
- Eisai Co., Ltd. (Japan)
- Genisphere LLC (USA)
- HaploX (USA)
- Kymab Limited (UK)
- Lonza Group AG (Switzerland)
- Merck KGaA (Germany)
- Peptide Therapeutics Limited (UK)
- Peptron (South Korea)
- Roche Holding AG (Switzerland)
- Sarepta Therapeutics, Inc. (USA)
- Thermo Fisher Scientific Inc. (USA)
- Trojan Peptide (USA)
- Other Active Players
Key Industry Developments in the Cell Penetrating Peptide Market:
- In May 2023, Tocris Bioscience introduced a new CPP-based siRNA delivery method. This technique, which is well-known for its remarkable efficiency, can deliver siRNA to a wide variety of cell types, providing substantial promise for gene therapy and molecular research.
|
Cell Penetrating Peptide Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 1.47 Billion |
|
Forecast Period 2024-32 CAGR: |
16.2 % |
Market Size in 2032: |
USD 7.15 Billion |
|
Segments Covered: |
By Type |
|
|
|
By Application |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Cell Penetrating Peptide Market by By Type (2018-2032)
4.1 Cell Penetrating Peptide Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Protein-based CPPs: TAT peptide
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 Penetratin
4.5 Antp
4.6 Peptide-based CPPs: Oligoarginine peptide
4.7 Polyarginine peptide
4.8 Transportan
Chapter 5: Cell Penetrating Peptide Market by By Application (2018-2032)
5.1 Cell Penetrating Peptide Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Drug Delivery
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Gene Delivery
5.5 Diagnostics
5.6 Molecular Imaging
Chapter 6: Cell Penetrating Peptide Market by By End User (2018-2032)
6.1 Cell Penetrating Peptide Market Snapshot and Growth Engine
6.2 Market Overview
6.3 Pharmaceutical and Biotechnology Companies
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
6.3.3 Key Market Trends, Growth Factors, and Opportunities
6.3.4 Geographic Segmentation Analysis
6.4 Contract Research Organizations (CROs)
6.5 Hospitals and Clinics
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 Cell Penetrating Peptide Market Share by Manufacturer (2024)
7.1.3 Industry BCG Matrix
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 AMINO LABS (USA)
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Key Strategic Moves and Recent Developments
7.2.10 SWOT Analysis
7.3 APEXIGEN INC. (USA)
7.4 BACHEM HOLDING AG (SWITZERLAND)
7.5 BIOMX INC. (ISRAEL)
7.6 CUREVAC AG (GERMANY)
7.7 CYTOMX THERAPEUTICS INC. (USA)
7.8 EISAI CO. LTD. (JAPAN)
7.9 GENISPHERE LLC (USA)
7.10 HAPLOX (USA)
7.11 KYMAB LIMITED (UK)
7.12 LONZA GROUP AG (SWITZERLAND)
7.13 MERCK KGAA (GERMANY)
7.14 PEPTIDE THERAPEUTICS LIMITED (UK)
7.15 PEPTRON (SOUTH KOREA)
7.16 ROCHE HOLDING AG (SWITZERLAND)
7.17 SAREPTA THERAPEUTICS INC. (USA)
7.18 THERMO FISHER SCIENTIFIC INC. (USA)
7.19 TROJAN PEPTIDE (USA)
7.20 OTHER ACTIVE PLAYERS
Chapter 8: Global Cell Penetrating Peptide Market By Region
8.1 Overview
8.2. North America Cell Penetrating Peptide Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecasted Market Size By By Type
8.2.4.1 Protein-based CPPs: TAT peptide
8.2.4.2 Penetratin
8.2.4.3 Antp
8.2.4.4 Peptide-based CPPs: Oligoarginine peptide
8.2.4.5 Polyarginine peptide
8.2.4.6 Transportan
8.2.5 Historic and Forecasted Market Size By By Application
8.2.5.1 Drug Delivery
8.2.5.2 Gene Delivery
8.2.5.3 Diagnostics
8.2.5.4 Molecular Imaging
8.2.6 Historic and Forecasted Market Size By By End User
8.2.6.1 Pharmaceutical and Biotechnology Companies
8.2.6.2 Contract Research Organizations (CROs)
8.2.6.3 Hospitals and Clinics
8.2.7 Historic and Forecast Market Size by Country
8.2.7.1 US
8.2.7.2 Canada
8.2.7.3 Mexico
8.3. Eastern Europe Cell Penetrating Peptide Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecasted Market Size By By Type
8.3.4.1 Protein-based CPPs: TAT peptide
8.3.4.2 Penetratin
8.3.4.3 Antp
8.3.4.4 Peptide-based CPPs: Oligoarginine peptide
8.3.4.5 Polyarginine peptide
8.3.4.6 Transportan
8.3.5 Historic and Forecasted Market Size By By Application
8.3.5.1 Drug Delivery
8.3.5.2 Gene Delivery
8.3.5.3 Diagnostics
8.3.5.4 Molecular Imaging
8.3.6 Historic and Forecasted Market Size By By End User
8.3.6.1 Pharmaceutical and Biotechnology Companies
8.3.6.2 Contract Research Organizations (CROs)
8.3.6.3 Hospitals and Clinics
8.3.7 Historic and Forecast Market Size by Country
8.3.7.1 Russia
8.3.7.2 Bulgaria
8.3.7.3 The Czech Republic
8.3.7.4 Hungary
8.3.7.5 Poland
8.3.7.6 Romania
8.3.7.7 Rest of Eastern Europe
8.4. Western Europe Cell Penetrating Peptide Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecasted Market Size By By Type
8.4.4.1 Protein-based CPPs: TAT peptide
8.4.4.2 Penetratin
8.4.4.3 Antp
8.4.4.4 Peptide-based CPPs: Oligoarginine peptide
8.4.4.5 Polyarginine peptide
8.4.4.6 Transportan
8.4.5 Historic and Forecasted Market Size By By Application
8.4.5.1 Drug Delivery
8.4.5.2 Gene Delivery
8.4.5.3 Diagnostics
8.4.5.4 Molecular Imaging
8.4.6 Historic and Forecasted Market Size By By End User
8.4.6.1 Pharmaceutical and Biotechnology Companies
8.4.6.2 Contract Research Organizations (CROs)
8.4.6.3 Hospitals and Clinics
8.4.7 Historic and Forecast Market Size by Country
8.4.7.1 Germany
8.4.7.2 UK
8.4.7.3 France
8.4.7.4 The Netherlands
8.4.7.5 Italy
8.4.7.6 Spain
8.4.7.7 Rest of Western Europe
8.5. Asia Pacific Cell Penetrating Peptide Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecasted Market Size By By Type
8.5.4.1 Protein-based CPPs: TAT peptide
8.5.4.2 Penetratin
8.5.4.3 Antp
8.5.4.4 Peptide-based CPPs: Oligoarginine peptide
8.5.4.5 Polyarginine peptide
8.5.4.6 Transportan
8.5.5 Historic and Forecasted Market Size By By Application
8.5.5.1 Drug Delivery
8.5.5.2 Gene Delivery
8.5.5.3 Diagnostics
8.5.5.4 Molecular Imaging
8.5.6 Historic and Forecasted Market Size By By End User
8.5.6.1 Pharmaceutical and Biotechnology Companies
8.5.6.2 Contract Research Organizations (CROs)
8.5.6.3 Hospitals and Clinics
8.5.7 Historic and Forecast Market Size by Country
8.5.7.1 China
8.5.7.2 India
8.5.7.3 Japan
8.5.7.4 South Korea
8.5.7.5 Malaysia
8.5.7.6 Thailand
8.5.7.7 Vietnam
8.5.7.8 The Philippines
8.5.7.9 Australia
8.5.7.10 New Zealand
8.5.7.11 Rest of APAC
8.6. Middle East & Africa Cell Penetrating Peptide Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecasted Market Size By By Type
8.6.4.1 Protein-based CPPs: TAT peptide
8.6.4.2 Penetratin
8.6.4.3 Antp
8.6.4.4 Peptide-based CPPs: Oligoarginine peptide
8.6.4.5 Polyarginine peptide
8.6.4.6 Transportan
8.6.5 Historic and Forecasted Market Size By By Application
8.6.5.1 Drug Delivery
8.6.5.2 Gene Delivery
8.6.5.3 Diagnostics
8.6.5.4 Molecular Imaging
8.6.6 Historic and Forecasted Market Size By By End User
8.6.6.1 Pharmaceutical and Biotechnology Companies
8.6.6.2 Contract Research Organizations (CROs)
8.6.6.3 Hospitals and Clinics
8.6.7 Historic and Forecast Market Size by Country
8.6.7.1 Turkiye
8.6.7.2 Bahrain
8.6.7.3 Kuwait
8.6.7.4 Saudi Arabia
8.6.7.5 Qatar
8.6.7.6 UAE
8.6.7.7 Israel
8.6.7.8 South Africa
8.7. South America Cell Penetrating Peptide Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecasted Market Size By By Type
8.7.4.1 Protein-based CPPs: TAT peptide
8.7.4.2 Penetratin
8.7.4.3 Antp
8.7.4.4 Peptide-based CPPs: Oligoarginine peptide
8.7.4.5 Polyarginine peptide
8.7.4.6 Transportan
8.7.5 Historic and Forecasted Market Size By By Application
8.7.5.1 Drug Delivery
8.7.5.2 Gene Delivery
8.7.5.3 Diagnostics
8.7.5.4 Molecular Imaging
8.7.6 Historic and Forecasted Market Size By By End User
8.7.6.1 Pharmaceutical and Biotechnology Companies
8.7.6.2 Contract Research Organizations (CROs)
8.7.6.3 Hospitals and Clinics
8.7.7 Historic and Forecast Market Size by Country
8.7.7.1 Brazil
8.7.7.2 Argentina
8.7.7.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
|
Cell Penetrating Peptide Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 1.47 Billion |
|
Forecast Period 2024-32 CAGR: |
16.2 % |
Market Size in 2032: |
USD 7.15 Billion |
|
Segments Covered: |
By Type |
|
|
|
By Application |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Frequently Asked Questions :
The forecast period in the Cell Penetrating Peptide Market research report is 2024-2032.
Amino Labs (USA), Apexigen, Inc. (USA), Bachem Holding AG (Switzerland), BiomX Inc. (Israel), CureVac AG (Germany), CytomX Therapeutics, Inc. (USA), Eisai Co., Ltd. (Japan), Genisphere LLC (USA), HaploX (USA), Kymab Limited (UK). and Other Major Players.
The Cell Penetrating Peptide Market is segmented into Type, Application, End User and region. By Type, the market is categorized into (Protein-based CPPs (TAT peptide, Penetratin, Antp),Peptide-based CPPs (Oligoarginine peptide, Polysrginine peptide, Transportan). By Application, the market is categorized into Drug Delivery,Gene Delivery,Diagnostics,Molecular Imaging). By End User, the market is categorized into Pharmaceutical and Biotechnology Company,Contract Research Organization (CROs),Hospitals and Clinics. By region, it is analyzed across North America (U.S., Canada, Mexico), Eastern Europe (Russia, Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe), Western Europe (Germany, UK, France, The Netherlands, Italy, Spain, Rest of Western Europe), Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New-Zealand, Rest of APAC), Middle East & Africa (Turkiye, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa), South America (Brazil, Argentina, Rest of SA).
The cell-penetrating peptide (CPP) market is a part of the biotechnology and pharmaceutical industries that is growing quickly. This is because more and more people need effective ways to deliver drugs, especially those that are hard for proteins, nucleic acids, and small molecules to cross cellular membranes. CPPs are short, often cationic peptides that help these medicinal agents get into cells. This makes them more bioavailable and effective while reducing their side effects. The market is growing because of progress in peptide synthesis, a greater focus on targeted treatments for long-term diseases like cancer and genetic disorders, and more money being put into biopharmaceutical research. Drug delivery, gene therapy, and vaccine development are some of the most important uses of CPPs. Pharmaceutical companies, university research institutions, and contract research organizations are all very interested in them. Still, problems like legal hurdles, stability, and bioavailability worries keep getting in the way of developing and selling CPP-based therapies. Overall, the CPP market is expected to keep growing as new technologies come out and more people look for new ways to treat illnesses.
Cell Penetrating Peptide Market Size Was Valued at USD 1.47 Billion in 2023, and is Projected to Reach USD 7.15 Billion by 2032, Growing at a CAGR of 16.2 % From 2024-2032.








