Clinical Trial Biorepository and Archiving Solutions Market Synopsis:
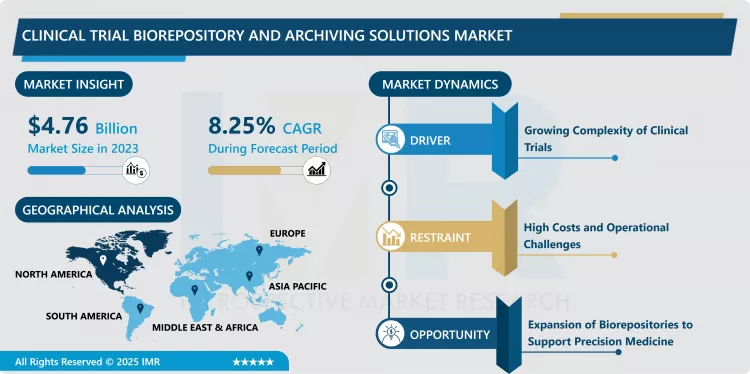
Clinical Trial Biorepository and Archiving Solutions Market Size Was Valued at USD 4.76 Billion in 2023, and is Projected to Reach USD 9.72 Billion by 2032, Growing at a CAGR of 8.25% From 2024-2032.
The Clinical Trial Biorepository and Archiving Solutions Market is a market who services and assets are used for sample storage, sample, data, and documentation management and archiving. It targets pharmaceutical industries, CROs and universities as they can use this solutions to store samples and records needed for research, compliance, archiving and future use in a clinical trial.
Clinical trials directly benefit from biorepositories by providing storage solutions for biological materials such as blood, tissues and DNA which are fundamentally required for future and on-going studies. Such points contribute to maintaining the integrity of samples because of controlled environment and security. Moreover, archiving solutions help with the proper storage and rational organization of trial information and paperwork to compliance with the rules. The high growth of clinical trials, involving more complex treatments, increased geographic distribution and inherent nature of biological material and clinical data to be stored requires more reliable and robust storage solutions. Hence, to meet regulatory requirements, protect samples from degradation and support storage of samples, companies are looking at superior biorepositories with better archiving services.
Additional propelling the market is the increasing relevance of PM and biomarker analyses, where samples obtained at a trial can be retested to find something else. The original archiving solutions should, therefore, meet the emerging requirements such as the GCP guidelines that are more stringent in ensuring that data is easily traceable and accurate. Increased expenditure in biorepositories and archiving services show signs of a transition to improved and conformant storage services which shows the significance of these services to serve as basis for further clinical studies and additional utilization of personalized disease management.

Clinical Trial Biorepository and Archiving Solutions Market Trend Analysis:
Rising Demand for Digital Biorepository Management Systems
- Emerging trends describe current biorepositories the complexity change for digital biorepository management systems that apply cloud computing, blockchain, and artificial intelligence (AI). Computers and specifically designed hardware platforms improve problem of sample identification, allow secure data exchange, and give access to the data and sample data repository with instant availability. Biorepositories use records and data in digitized forms, which means that sample data is well managed and accounts for its accessibility, traceability while at the same time maintaining high integrity levels which makes it easier to respond to such issues of compliance.
- This kind of solutions proves especially helpful in cases of multination wide clinical trials during which samples are collected from all over the world and constant communication is vital. Cloud-based storage has a capability for growth and flexibility which means that researchers and institutions can access data from different locations. These systems also include artificial intelligence for supporting researchers in analyzing stored data for patterns or for signs that indicate any abnormality; these characteristics provide more meaning and usage of biological repositories other than for storage and reference only, but for results- oriented, data-analyzing, and, hence, research.
Expansion of Biorepositories to Support Precision Medicine
- The advent of the new model of precision medicine in disease diagnosis and treatment based on the consumer’s genetic, environmental and lifestyle profile therefore opens a very attractive future for the biorepository market. Precision medicine thus explicitly depends on the availability of very good quality biospecimens for biomarker discovery and subsequent targeted therapeutics. Consequently, the need for biorepositories capable of processing genetic and molecular samples is increasing. Biorepositories should be able to advance this trend by enhancing their capabilities for specific storage and depot that there is construction for genomics and proteomics samples.
- As pharmaceutical companies and the institutions conduct research in precision medicine, biorepositories have a chance of taking up more business and offer more services. They assist in becoming invaluable for precision medicine by offering specialized storage systems that are specifically geared toward biomarker research, and genetics. This expansion also permits partnering with precision medicine researchers, which may open up future collaborations spanning sample storage alongside analysis leading several breakthroughs in individualised treatment approaches.
Clinical Trial Biorepository and Archiving Solutions Market Segment Analysis:
Clinical Trial Biorepository and Archiving Solutions Market is Segmented on the basis of Type, Application, End User, and Region
By Service Type, Biorepository Services segment is expected to dominate the market during the forecast period
- Biorepository services include a process of safe containment of biologic samples; samples need to be stored in conditions that do not allow samples deterioration. These services are required to maintain biological samples for a relatively long time which is important in clinical research and any other project that requires samples to be preserved for a long time. While on the other it pertains to services offered for archiving clinical trial documentation and information. These solutions are crucial for compliance purposes as the storage meets the necessary standards relative to data storage and offers structures for storage that allow for tracking, as well as access to important information.
- While biorepository services as a whole deal with the storage and management of tissues or bio-specimens, archiving services focus more on compliance and accuracy of information. Remarkably enough, the volume of trial data collected is massive and proper safe and secure record storage is crucial. ESS operates independently yet are symbiotic and coopervate with QRS in supporting clinical research throughout its trial by providing sample storage and meeting regulatory compliance.
By End User, Pharmaceutical & Biotechnology Companies segment expected to held the largest share
- Biotechnology and pharmaceuticals is one of the largest client segments for biorepository and archiving since drugs and biologics candidates involve collection of samples during the development of the drug. Contract research organizations (CROs) also form a significant part as they are involved in the running of trials on behalf of their pharmaceutical client by availing best storage solutions for samples and data. These services are applied in hospitals and clinical laboratories to store patients material for use in diagnosis and research now and in the future.
- For their part, smaller institutions are still emerging as key clients for biorepositories since the institutions offer large-scale research services, many of which entail the storage of biological specimens for lengthy research projects. Every single end user of biorepository and archiving services has individual storage requirements – from regulatory compliance in trade commercial clinical trials to sample accessibility in continuous academic research.
Clinical Trial Biorepository and Archiving Solutions Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
- North America dominates the Clinical Trial Biorepository and Archiving Solutions Market because of its advanced healthcare systems, a large number of ongoing clinical trials and adherence to stringent regulatory standards. The region is also characterized by strong manufacturing base of pharmaceutical companies and research institutions; many of which are considered to be the most important customers of biorepository and archiving services. Moreover, the regional and federal regulations such as FDA in North America has very strict rules or samples and different data of the biorepositories leading to the need for proper biorepository solutions.
- Further, North America has a large focus toward the research and development which increases the demand for sophisticated biorepository services. As more capital is directed towards precision medicine and clinical trial solutions, this region remains committed in acquiring storage forms with compliance, quality, and retrievability. Thus, North America continues to be the key market for bio samples and biorepositories while establishing a set of global industry standards for sample storage.
Active Key Players in the Clinical Trial Biorepository and Archiving Solutions Market:
- Thermo Fisher Scientific Inc. (USA)
- Labcorp Drug Development (USA)
- Precision for Medicine (USA)
- Q2 Solutions (USA)
- Charles River Laboratories (USA)
- ICON plc (Ireland)
- Medpace, Inc. (USA)
- BioStorage Technologies (USA)
- Parexel International Corporation (USA)
- BioIVT (USA)
- Covance Inc. (USA)
- WuXi AppTec (China)
- Other Active Players
Key Industry Developments in the Clinical Trial Biorepository and Archiving Solutions Market:
- In June 2024, Cryoport expanded its Pont-du-Chateau facility in France. This expansion more than doubled the facility's capacity, enhancing its biostorage and bioservice capabilities.
- In April 2024, LabConnect acquired a scientific consulting company, A4P Consulting Ltd (A4P). A4P Consulting Ltd specializes in biomarker strategy, biosample & bioanalytical project management, and logistics solutions for preclinical & clinical trials.
|
Global Clinical Trial Biorepository and Archiving Solutions Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 4.76 Billion |
|
Forecast Period 2024-32 CAGR: |
8.25% |
Market Size in 2032: |
USD 9.72 Billion |
|
Segments Covered: |
By Service Type |
|
|
|
By Phase |
|
||
|
By Biospecimen Type |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Clinical Trial Biorepository and Archiving Solutions Market by By Service Type (2018-2032)
4.1 Clinical Trial Biorepository and Archiving Solutions Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Biorepository Services
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 Archiving Services
Chapter 5: Clinical Trial Biorepository and Archiving Solutions Market by By Phase (2018-2032)
5.1 Clinical Trial Biorepository and Archiving Solutions Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Preclinical
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Phase I
5.5 Phase II
5.6 Phase III
5.7 Phase IV
Chapter 6: Clinical Trial Biorepository and Archiving Solutions Market by By Biospecimen Type (2018-2032)
6.1 Clinical Trial Biorepository and Archiving Solutions Market Snapshot and Growth Engine
6.2 Market Overview
6.3 Solid Tissue
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
6.3.3 Key Market Trends, Growth Factors, and Opportunities
6.3.4 Geographic Segmentation Analysis
6.4 Biofluids
6.5 Nucleic Acids
6.6 Cell Lines
6.7 Other
Chapter 7: Clinical Trial Biorepository and Archiving Solutions Market by By End User (2018-2032)
7.1 Clinical Trial Biorepository and Archiving Solutions Market Snapshot and Growth Engine
7.2 Market Overview
7.3 Pharmaceutical & Biotechnology Companies
7.3.1 Introduction and Market Overview
7.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
7.3.3 Key Market Trends, Growth Factors, and Opportunities
7.3.4 Geographic Segmentation Analysis
7.4 Academic & Research Institutions
7.5 Contract Research Organizations (CROs)
7.6 Hospitals & Clinical Labs
Chapter 8: Company Profiles and Competitive Analysis
8.1 Competitive Landscape
8.1.1 Competitive Benchmarking
8.1.2 Clinical Trial Biorepository and Archiving Solutions Market Share by Manufacturer (2024)
8.1.3 Industry BCG Matrix
8.1.4 Heat Map Analysis
8.1.5 Mergers and Acquisitions
8.2 THERMO FISHER SCIENTIFIC INC. (USA)
8.2.1 Company Overview
8.2.2 Key Executives
8.2.3 Company Snapshot
8.2.4 Role of the Company in the Market
8.2.5 Sustainability and Social Responsibility
8.2.6 Operating Business Segments
8.2.7 Product Portfolio
8.2.8 Business Performance
8.2.9 Key Strategic Moves and Recent Developments
8.2.10 SWOT Analysis
8.3 LABCORP DRUG DEVELOPMENT (USA)
8.4 PRECISION FOR MEDICINE (USA)
8.5 Q2 SOLUTIONS (USA)
8.6 CHARLES RIVER LABORATORIES (USA)
8.7 ICON PLC (IRELAND)
8.8 MEDPACE INC. (USA)
8.9 BIOSTORAGE TECHNOLOGIES (USA)
8.10 OTHER ACTIVE PLAYERS
Chapter 9: Global Clinical Trial Biorepository and Archiving Solutions Market By Region
9.1 Overview
9.2. North America Clinical Trial Biorepository and Archiving Solutions Market
9.2.1 Key Market Trends, Growth Factors and Opportunities
9.2.2 Top Key Companies
9.2.3 Historic and Forecasted Market Size by Segments
9.2.4 Historic and Forecasted Market Size By By Service Type
9.2.4.1 Biorepository Services
9.2.4.2 Archiving Services
9.2.5 Historic and Forecasted Market Size By By Phase
9.2.5.1 Preclinical
9.2.5.2 Phase I
9.2.5.3 Phase II
9.2.5.4 Phase III
9.2.5.5 Phase IV
9.2.6 Historic and Forecasted Market Size By By Biospecimen Type
9.2.6.1 Solid Tissue
9.2.6.2 Biofluids
9.2.6.3 Nucleic Acids
9.2.6.4 Cell Lines
9.2.6.5 Other
9.2.7 Historic and Forecasted Market Size By By End User
9.2.7.1 Pharmaceutical & Biotechnology Companies
9.2.7.2 Academic & Research Institutions
9.2.7.3 Contract Research Organizations (CROs)
9.2.7.4 Hospitals & Clinical Labs
9.2.8 Historic and Forecast Market Size by Country
9.2.8.1 US
9.2.8.2 Canada
9.2.8.3 Mexico
9.3. Eastern Europe Clinical Trial Biorepository and Archiving Solutions Market
9.3.1 Key Market Trends, Growth Factors and Opportunities
9.3.2 Top Key Companies
9.3.3 Historic and Forecasted Market Size by Segments
9.3.4 Historic and Forecasted Market Size By By Service Type
9.3.4.1 Biorepository Services
9.3.4.2 Archiving Services
9.3.5 Historic and Forecasted Market Size By By Phase
9.3.5.1 Preclinical
9.3.5.2 Phase I
9.3.5.3 Phase II
9.3.5.4 Phase III
9.3.5.5 Phase IV
9.3.6 Historic and Forecasted Market Size By By Biospecimen Type
9.3.6.1 Solid Tissue
9.3.6.2 Biofluids
9.3.6.3 Nucleic Acids
9.3.6.4 Cell Lines
9.3.6.5 Other
9.3.7 Historic and Forecasted Market Size By By End User
9.3.7.1 Pharmaceutical & Biotechnology Companies
9.3.7.2 Academic & Research Institutions
9.3.7.3 Contract Research Organizations (CROs)
9.3.7.4 Hospitals & Clinical Labs
9.3.8 Historic and Forecast Market Size by Country
9.3.8.1 Russia
9.3.8.2 Bulgaria
9.3.8.3 The Czech Republic
9.3.8.4 Hungary
9.3.8.5 Poland
9.3.8.6 Romania
9.3.8.7 Rest of Eastern Europe
9.4. Western Europe Clinical Trial Biorepository and Archiving Solutions Market
9.4.1 Key Market Trends, Growth Factors and Opportunities
9.4.2 Top Key Companies
9.4.3 Historic and Forecasted Market Size by Segments
9.4.4 Historic and Forecasted Market Size By By Service Type
9.4.4.1 Biorepository Services
9.4.4.2 Archiving Services
9.4.5 Historic and Forecasted Market Size By By Phase
9.4.5.1 Preclinical
9.4.5.2 Phase I
9.4.5.3 Phase II
9.4.5.4 Phase III
9.4.5.5 Phase IV
9.4.6 Historic and Forecasted Market Size By By Biospecimen Type
9.4.6.1 Solid Tissue
9.4.6.2 Biofluids
9.4.6.3 Nucleic Acids
9.4.6.4 Cell Lines
9.4.6.5 Other
9.4.7 Historic and Forecasted Market Size By By End User
9.4.7.1 Pharmaceutical & Biotechnology Companies
9.4.7.2 Academic & Research Institutions
9.4.7.3 Contract Research Organizations (CROs)
9.4.7.4 Hospitals & Clinical Labs
9.4.8 Historic and Forecast Market Size by Country
9.4.8.1 Germany
9.4.8.2 UK
9.4.8.3 France
9.4.8.4 The Netherlands
9.4.8.5 Italy
9.4.8.6 Spain
9.4.8.7 Rest of Western Europe
9.5. Asia Pacific Clinical Trial Biorepository and Archiving Solutions Market
9.5.1 Key Market Trends, Growth Factors and Opportunities
9.5.2 Top Key Companies
9.5.3 Historic and Forecasted Market Size by Segments
9.5.4 Historic and Forecasted Market Size By By Service Type
9.5.4.1 Biorepository Services
9.5.4.2 Archiving Services
9.5.5 Historic and Forecasted Market Size By By Phase
9.5.5.1 Preclinical
9.5.5.2 Phase I
9.5.5.3 Phase II
9.5.5.4 Phase III
9.5.5.5 Phase IV
9.5.6 Historic and Forecasted Market Size By By Biospecimen Type
9.5.6.1 Solid Tissue
9.5.6.2 Biofluids
9.5.6.3 Nucleic Acids
9.5.6.4 Cell Lines
9.5.6.5 Other
9.5.7 Historic and Forecasted Market Size By By End User
9.5.7.1 Pharmaceutical & Biotechnology Companies
9.5.7.2 Academic & Research Institutions
9.5.7.3 Contract Research Organizations (CROs)
9.5.7.4 Hospitals & Clinical Labs
9.5.8 Historic and Forecast Market Size by Country
9.5.8.1 China
9.5.8.2 India
9.5.8.3 Japan
9.5.8.4 South Korea
9.5.8.5 Malaysia
9.5.8.6 Thailand
9.5.8.7 Vietnam
9.5.8.8 The Philippines
9.5.8.9 Australia
9.5.8.10 New Zealand
9.5.8.11 Rest of APAC
9.6. Middle East & Africa Clinical Trial Biorepository and Archiving Solutions Market
9.6.1 Key Market Trends, Growth Factors and Opportunities
9.6.2 Top Key Companies
9.6.3 Historic and Forecasted Market Size by Segments
9.6.4 Historic and Forecasted Market Size By By Service Type
9.6.4.1 Biorepository Services
9.6.4.2 Archiving Services
9.6.5 Historic and Forecasted Market Size By By Phase
9.6.5.1 Preclinical
9.6.5.2 Phase I
9.6.5.3 Phase II
9.6.5.4 Phase III
9.6.5.5 Phase IV
9.6.6 Historic and Forecasted Market Size By By Biospecimen Type
9.6.6.1 Solid Tissue
9.6.6.2 Biofluids
9.6.6.3 Nucleic Acids
9.6.6.4 Cell Lines
9.6.6.5 Other
9.6.7 Historic and Forecasted Market Size By By End User
9.6.7.1 Pharmaceutical & Biotechnology Companies
9.6.7.2 Academic & Research Institutions
9.6.7.3 Contract Research Organizations (CROs)
9.6.7.4 Hospitals & Clinical Labs
9.6.8 Historic and Forecast Market Size by Country
9.6.8.1 Turkiye
9.6.8.2 Bahrain
9.6.8.3 Kuwait
9.6.8.4 Saudi Arabia
9.6.8.5 Qatar
9.6.8.6 UAE
9.6.8.7 Israel
9.6.8.8 South Africa
9.7. South America Clinical Trial Biorepository and Archiving Solutions Market
9.7.1 Key Market Trends, Growth Factors and Opportunities
9.7.2 Top Key Companies
9.7.3 Historic and Forecasted Market Size by Segments
9.7.4 Historic and Forecasted Market Size By By Service Type
9.7.4.1 Biorepository Services
9.7.4.2 Archiving Services
9.7.5 Historic and Forecasted Market Size By By Phase
9.7.5.1 Preclinical
9.7.5.2 Phase I
9.7.5.3 Phase II
9.7.5.4 Phase III
9.7.5.5 Phase IV
9.7.6 Historic and Forecasted Market Size By By Biospecimen Type
9.7.6.1 Solid Tissue
9.7.6.2 Biofluids
9.7.6.3 Nucleic Acids
9.7.6.4 Cell Lines
9.7.6.5 Other
9.7.7 Historic and Forecasted Market Size By By End User
9.7.7.1 Pharmaceutical & Biotechnology Companies
9.7.7.2 Academic & Research Institutions
9.7.7.3 Contract Research Organizations (CROs)
9.7.7.4 Hospitals & Clinical Labs
9.7.8 Historic and Forecast Market Size by Country
9.7.8.1 Brazil
9.7.8.2 Argentina
9.7.8.3 Rest of SA
Chapter 10 Analyst Viewpoint and Conclusion
10.1 Recommendations and Concluding Analysis
10.2 Potential Market Strategies
Chapter 11 Research Methodology
11.1 Research Process
11.2 Primary Research
11.3 Secondary Research
|
Global Clinical Trial Biorepository and Archiving Solutions Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 4.76 Billion |
|
Forecast Period 2024-32 CAGR: |
8.25% |
Market Size in 2032: |
USD 9.72 Billion |
|
Segments Covered: |
By Service Type |
|
|
|
By Phase |
|
||
|
By Biospecimen Type |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Frequently Asked Questions :
The forecast period in the Clinical Trial Biorepository and Archiving Solutions Market research report is 2024-2032.
Thermo Fisher Scientific Inc. (USA), Labcorp Drug Development (USA), Precision for Medicine (USA), Q2 Solutions (USA), Charles River Laboratories (USA), ICON plc (Ireland), Medpace, Inc. (USA), BioStorage Technologies (USA), Parexel International Corporation (USA), BioIVT (USA), Covance Inc. (USA), WuXi AppTec (China), and Other Active Players.
The Clinical Trial Biorepository and Archiving Solutions Market is segmented into Service Type, Phase, Biospecimen Type, End User and region. By Service Type, the market is categorized into Biorepository Services, Archiving Services. By Phase, the market is categorized into Preclinical, Phase I, Phase II, Phase III, Phase IV. By Biospecimen Type, the market is categorized into Solid Tissue, Biofluids, Nucleic Acids, Cell Lines, Other. By End User, the market is categorized into Pharmaceutical & Biotechnology Companies, Academic & Research Institutions, Contract Research Organizations (CROs), Hospitals & Clinical Labs. By region, it is analyzed across North America (U.S., Canada, Mexico), Eastern Europe (Russia, Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe), Western Europe (Germany, UK, France, The Netherlands, Italy, Spain, Rest of Western Europe), Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New-Zealand, Rest of APAC), Middle East & Africa (Turkiye, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa), South America (Brazil, Argentina, Rest of SA).
The Clinical Trial Biorepository and Archiving Solutions Market is a market who services and assets are used for sample storage, sample, data, and documentation management and archiving. It targets pharmaceutical industries, CROs and universities as they can use this solutions to store samples and records needed for research, compliance, archiving and future use in a clinical trial.
Clinical Trial Biorepository and Archiving Solutions Market Size Was Valued at USD 4.76 Billion in 2023, and is Projected to Reach USD 9.72 Billion by 2032, Growing at a CAGR of 8.25% From 2024-2032.








