Clinical Trial Management System Market Synopsis:
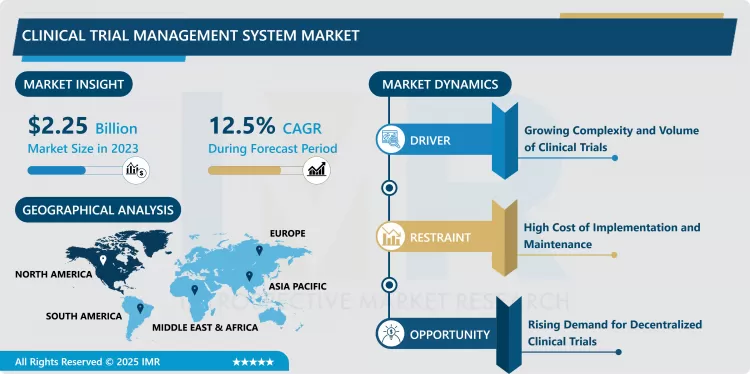
Clinical Trial Management System Market Size Was Valued at USD 2.25 Billion in 2023, and is Projected to Reach USD 6.49 Billion by 2032, Growing at a CAGR of 12.5 % From 2024-2032.
The CTMS market is a specialty segment for those clinically based solutions, interactive management, and tracking tools for clinical trials and the processes associated with them. This market is informed by the need for efficient, accurate, and centralized solutions to various actors in the clinical trial value chain, including pharma companies, research organizations and CROs. From participant recognition and appointment to monetary control and preparation and submission of administrative documents, CTMS solutions are beneficial in relation to the coordination of the several sites, parties, and phases of a trial. With this demand, the evolution of such systems remains a thriving area because each part of the trial’s life cycle benefits from the growing opportunities for translating Hedderman and enhancing data openness in general.
As the result of an increasing number of clinical trials and more stringent regulations the Clinical Trial Management System market is likely to grow with a desired rate in the following years. CROs are in desperate need of effective software that will improve data management, adherence to established standards and co-ordination of several and at times cross-continental groups. As these processes are generally time consuming, the CTMS platforms also manage budget distribution, site performance and data integration better which is extremely important for the success of large multi-site trials. Thirdly, advancements in diseases’ nature, including rising incidences of chronic diseases, need for individualized pharmacological therapies, and growing pressure on pharmaceutical companies to up the pace for discovering new drugs, facilitated the adoption of CTMS in recent years that have gained significant significance for clinical research.
Not only that, but the continual advancement of digital technology has also dictated the nature of the CTMS market and the boundaries within which it must work. Artificial intelligence or commonly referred to as AI, machine learning and cloud-based information’s are changing the-clinical data processing and management as well as distribution amongst actors. For instance, with implementation of Machine learning in CTMS, trial data can be tracked almost in real time, improving decision making on trial strategy and execution. Further, the decentralization of the clinical trials that began due to the pandemic has emphasized the need for a resilient CTMS suited to decentralized and virtually coordinated trials. These changes show that the CTMS Market is set for vibrant growth in the future of clinical research by improving co-ordination and compliance.

Clinical Trial Management System Market Trend Analysis:
Adoption of Cloud-Based CTMS Solutions for Enhanced Flexibility
- The most popular trends in the current CTMS market are presented based in the cloud solutions. A new trend is fixed with the gradual substitution of the traditional on-site products on the fast-growing cloud solutions that, to some extent, have some advantages over the former. This makes cloud-hosted systems appropriate for use in global trials, as well as in decentralized trial plans, since one may access the CTMS data in real time from almost any location. These solutions enable some remote parts of a clinical trial process and provide better synchronizing, integration with other technologies, and implementation compared to traditional methods. The problem has thus emerged to seek solutions that can easily manage new trial protocols and conditions as well as frequent changes of the regulations. Further, the changed security enhancement in cloud technology addresses concerns on data security, which consequently has made trial management easier for organizations particularly those keen on regaining improved compliance standards.
Growing Demand for Decentralized Clinical Trials
- The increasing need for decentralized clinical trials (DCTs) holds one of the largest development opportunities in the CTMS market currently. With the changes in environment of clinical research, the consideration involving the reduction of the necessary distances for subjects to travel to clinical sites is apparent across every conceivable community to make it more patient friendly and efficient. The solutions to CTMS for DCT receive, review/revise e-PRA/PROs, performing telemedicine visits which improve a participant site access and retention. Another related trend is that, as with DCTs, incorporating integration with mHealth devices, EHRs and telehealth into the context of CTMS has also become significant. Those organisations who are able to install these capabilities as part of their CTMS platforms are likely to be best placed to control this market because at some point in the future, regulation authorities will offer the necessary incentive for DCT operation. This change also presents a good business opportunity for CTMS vendors since the current acclaimed innovation requirements of clinical trials are unmatched by what the vendors offer.
Clinical Trial Management System Market Segment Analysis:
Clinical Trial Management System Market is Segmented on the basis of Mode of Delivery, Component, End User, and Region
By Mode of delivery, cloud-based segment is expected to dominate the market during the forecast period
- Based on the mode of delivery, the cloud-based segment is anticipated to have the largest market share for the Clinical Trial Management System (CTMS) throughout the forecast period. We saw that there is a great adoption of cloud-based CTMS solutions because of their flexibility, affordability, and ease of use. One of the shores of cloud-based systems is that the user can gain data from anywhere and multiple trials sites wit support the increasing trend of decentralized & virtual trials. These platforms involve comparatively lower capital requirements in terms of information technology gear and hence entail lower expenses in terms of maintenance and servicing to boot; such platforms are equally advantageous for diverse entities ranging from small to medium-sized organizations and excluding only large business formation corporations. In the same way, some of the drawbacks associated with possession of big data such as security and compliance have received solutions within cloud technology making cloud solutions a favourable option for the companies which are in search for flexibility and viability within clinical trial management.
By Component, software segment expected to held the largest share
- According to the components, the software segment is expected to lead the clinical trial management system (CTMS) market through the forecast period. This has been witnessed revealing that the number of organizations is increasing for better and more comprehensive requirements to operate CTMS software that is require for digital clinical trial patient registration or recruitment tracking, sustain performance for continued compliance and reporting. Aids to make sense of the immense volumes of trial data and organize the data stream so that trials become less complicated and faster through CTMS software solutions. The more there are decentralized and virtual trials – the more there is reliance on software to manage trial activities, trial sites, regulatory agencies, and others. Also, improvements in the functionalities of CTMS software features such as AIS, Data analytics, and EHR integration place CTMS software on the solid foundation of clinical research. Because of the dependency on advanced software applications in the course of optimizing the trial processes in organizations, the main staying point of the software portion is going to continue being huge within the CTMS industry
Clinical Trial Management System Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
- North America is expected to gain the highest revenue in the Clinical Trial Management System (CTMS) market during the forecast period due to its established clinical trial system, and gross research and development spending as well as the increased presence of prominent pharmaceutical and biotechnology companies. Particular attention is paid to the United States due to legal regulation, multidisciplinary application of legislation to the field, significant investment in clinical research in various therapeutic areas, such as oncology, orphan diseases, and personalized medicine. trial management science is well-developed in the region, the density of highly qualified Trial professionals is high, and the region boasts of advanced trials technology and management systems; the region can be indexed as a world class area for ad tricky trials.
- This is also driving the North American market to a more commanding position in the context of CTMS market due to enhanced usage of as digital technology including artificial intelligence, machine learning and cloud computing offering better data analysis, remote trial monitoring and efficient communication instruments. As well Clinical trials and decentralized clinical trials have as well enhanced the need for flexible smart and agile CTMS not only for managing data from other source but also for the biospecimen management of remote patient. North American organizations are particularly prepared to adopt next generations of CTMS solutions to address new/regulatory change and improve the efficiency of trials that makes North America a center of innovative CTMS technologies. As long as more and more fund is put into clinical research and another closely related virtual trial goes on refining, North America’s leading status in the global CTMS market will not only keep stable but even strengthen for the whole course of the forecast period.
Active Key Players in the Clinical Trial Management System Market:
- Advarra (USA)
- ArisGlobal (USA)
- Bioclinica (USA)
- Bio-Optronics (USA)
- Clario (USA)
- Dassault Systèmes (France)
- eClinicalWorks (USA)
- Medidata Solutions (USA)
- MedNet Solutions (USA)
- Oracle Corporation (USA)
- Parexel International (USA)
- RealTime Software Solutions (USA)
- Saama Technologies (USA)
- Veeva Systems (USA)
- WuXi AppTec (China), Other Active Players.
|
Global Clinical Trial Management System Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 2.25 Billion |
|
Forecast Period 2024-32 CAGR: |
12.5% |
Market Size in 2032: |
USD 6.49 Billion |
|
Segments Covered: |
By Mode of Delivery |
|
|
|
By Component |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Clinical Trial Management System Market by By Mode of Delivery (2018-2032)
4.1 Clinical Trial Management System Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Software
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 Services
Chapter 5: Clinical Trial Management System Market by By Component (2018-2032)
5.1 Clinical Trial Management System Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Cloud base
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 On-Premise
Chapter 6: Clinical Trial Management System Market by By End User (2018-2032)
6.1 Clinical Trial Management System Market Snapshot and Growth Engine
6.2 Market Overview
6.3 Healthcare Companies
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
6.3.3 Key Market Trends, Growth Factors, and Opportunities
6.3.4 Geographic Segmentation Analysis
6.4 Biopharmaceutical companies
6.5 Clinical Research
6.6 Organization
6.7 Academic & Research Institutes
6.8 Others
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 Clinical Trial Management System Market Share by Manufacturer (2024)
7.1.3 Industry BCG Matrix
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 ADVARA (USA)
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Key Strategic Moves and Recent Developments
7.2.10 SWOT Analysis
7.3 ARIS GLOBAL (USA)
7.4 BAROCLINIC (USA)
7.5 BIO-OPTRONICS (USA)CLARIO (USA)
7.6 OTHER ACTIVE PLAYERS
Chapter 8: Global Clinical Trial Management System Market By Region
8.1 Overview
8.2. North America Clinical Trial Management System Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecasted Market Size By By Mode of Delivery
8.2.4.1 Software
8.2.4.2 Services
8.2.5 Historic and Forecasted Market Size By By Component
8.2.5.1 Cloud base
8.2.5.2 On-Premise
8.2.6 Historic and Forecasted Market Size By By End User
8.2.6.1 Healthcare Companies
8.2.6.2 Biopharmaceutical companies
8.2.6.3 Clinical Research
8.2.6.4 Organization
8.2.6.5 Academic & Research Institutes
8.2.6.6 Others
8.2.7 Historic and Forecast Market Size by Country
8.2.7.1 US
8.2.7.2 Canada
8.2.7.3 Mexico
8.3. Eastern Europe Clinical Trial Management System Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecasted Market Size By By Mode of Delivery
8.3.4.1 Software
8.3.4.2 Services
8.3.5 Historic and Forecasted Market Size By By Component
8.3.5.1 Cloud base
8.3.5.2 On-Premise
8.3.6 Historic and Forecasted Market Size By By End User
8.3.6.1 Healthcare Companies
8.3.6.2 Biopharmaceutical companies
8.3.6.3 Clinical Research
8.3.6.4 Organization
8.3.6.5 Academic & Research Institutes
8.3.6.6 Others
8.3.7 Historic and Forecast Market Size by Country
8.3.7.1 Russia
8.3.7.2 Bulgaria
8.3.7.3 The Czech Republic
8.3.7.4 Hungary
8.3.7.5 Poland
8.3.7.6 Romania
8.3.7.7 Rest of Eastern Europe
8.4. Western Europe Clinical Trial Management System Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecasted Market Size By By Mode of Delivery
8.4.4.1 Software
8.4.4.2 Services
8.4.5 Historic and Forecasted Market Size By By Component
8.4.5.1 Cloud base
8.4.5.2 On-Premise
8.4.6 Historic and Forecasted Market Size By By End User
8.4.6.1 Healthcare Companies
8.4.6.2 Biopharmaceutical companies
8.4.6.3 Clinical Research
8.4.6.4 Organization
8.4.6.5 Academic & Research Institutes
8.4.6.6 Others
8.4.7 Historic and Forecast Market Size by Country
8.4.7.1 Germany
8.4.7.2 UK
8.4.7.3 France
8.4.7.4 The Netherlands
8.4.7.5 Italy
8.4.7.6 Spain
8.4.7.7 Rest of Western Europe
8.5. Asia Pacific Clinical Trial Management System Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecasted Market Size By By Mode of Delivery
8.5.4.1 Software
8.5.4.2 Services
8.5.5 Historic and Forecasted Market Size By By Component
8.5.5.1 Cloud base
8.5.5.2 On-Premise
8.5.6 Historic and Forecasted Market Size By By End User
8.5.6.1 Healthcare Companies
8.5.6.2 Biopharmaceutical companies
8.5.6.3 Clinical Research
8.5.6.4 Organization
8.5.6.5 Academic & Research Institutes
8.5.6.6 Others
8.5.7 Historic and Forecast Market Size by Country
8.5.7.1 China
8.5.7.2 India
8.5.7.3 Japan
8.5.7.4 South Korea
8.5.7.5 Malaysia
8.5.7.6 Thailand
8.5.7.7 Vietnam
8.5.7.8 The Philippines
8.5.7.9 Australia
8.5.7.10 New Zealand
8.5.7.11 Rest of APAC
8.6. Middle East & Africa Clinical Trial Management System Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecasted Market Size By By Mode of Delivery
8.6.4.1 Software
8.6.4.2 Services
8.6.5 Historic and Forecasted Market Size By By Component
8.6.5.1 Cloud base
8.6.5.2 On-Premise
8.6.6 Historic and Forecasted Market Size By By End User
8.6.6.1 Healthcare Companies
8.6.6.2 Biopharmaceutical companies
8.6.6.3 Clinical Research
8.6.6.4 Organization
8.6.6.5 Academic & Research Institutes
8.6.6.6 Others
8.6.7 Historic and Forecast Market Size by Country
8.6.7.1 Turkiye
8.6.7.2 Bahrain
8.6.7.3 Kuwait
8.6.7.4 Saudi Arabia
8.6.7.5 Qatar
8.6.7.6 UAE
8.6.7.7 Israel
8.6.7.8 South Africa
8.7. South America Clinical Trial Management System Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecasted Market Size By By Mode of Delivery
8.7.4.1 Software
8.7.4.2 Services
8.7.5 Historic and Forecasted Market Size By By Component
8.7.5.1 Cloud base
8.7.5.2 On-Premise
8.7.6 Historic and Forecasted Market Size By By End User
8.7.6.1 Healthcare Companies
8.7.6.2 Biopharmaceutical companies
8.7.6.3 Clinical Research
8.7.6.4 Organization
8.7.6.5 Academic & Research Institutes
8.7.6.6 Others
8.7.7 Historic and Forecast Market Size by Country
8.7.7.1 Brazil
8.7.7.2 Argentina
8.7.7.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
|
Global Clinical Trial Management System Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 2.25 Billion |
|
Forecast Period 2024-32 CAGR: |
12.5% |
Market Size in 2032: |
USD 6.49 Billion |
|
Segments Covered: |
By Mode of Delivery |
|
|
|
By Component |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Frequently Asked Questions :
The forecast period in the Clinical Trial Management System Market research report is 2024-2032.
Advarra (USA), ArisGlobal (USA), Bioclinica (USA), Bio-Optronics (USA), Clario (USA), Dassault Systèmes (France), eClinicalWorks (USA), Medidata Solutions (USA), MedNet Solutions (USA), Oracle Corporation (USA), Parexel International (USA), RealTime Software Solutions (USA), Saama Technologies (USA), Veeva Systems (USA), WuXi AppTec (China), and Other Active Players.
The Clinical Trial Management System Market is segmented into Mode of Delivery, Component, End User and Region. By Mode of Delivery, the market is categorized into Cloud base and On-Premise4. By Component, the market is categorized into Software and services. By End User, the market is categorized into Healthcare Companies, Biopharmaceutical companies, Clinical Research, Organizations, Academic & Research Institutes, Others. By region, it is analyzed across North America (U.S., Canada, Mexico), Eastern Europe (Russia, Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe), Western Europe (Germany, UK, France, The Netherlands, Italy, Spain, Rest of Western Europe), Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New-Zealand, Rest of APAC), Middle East & Africa (Turkiye, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa), South America (Brazil, Argentina, Rest of SA).
The CTMS market is a specialty segment for those clinically based solutions, interactive management, and tracking tools for clinical trials and the processes associated with them. This market is informed by the need for efficient, accurate, and centralized solutions to various actors in the clinical trial value chain, including pharma companies, research organizations and CRO. From participant recognition and appointment to monetary control and preparation and submission of administrative documents, CTMS solutions are beneficial in relation to the coordination of the several sites, parties, and phases of a trial. With this demand, the evolution of such systems remains a thriving area because each part of the trial’s life cycle benefits from the growing opportunities for translating Hedderman and enhancing data openness in general
Clinical Trial Management System Market Size Was Valued at USD 2.25 Billion in 2023, and is Projected to Reach USD 6.49 Billion by 2032, Growing at a CAGR of 12.5 % From 2024-2032.








