Clostridium Difficile Diagnostics and Treatment Market Synopsis:
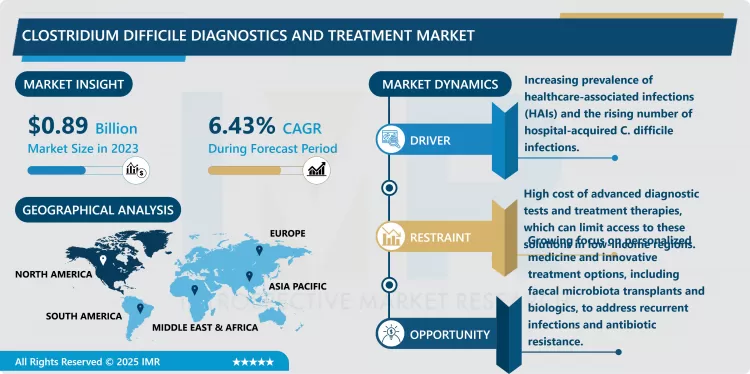
Clostridium Difficile Diagnostics and Treatment Market Size Was Valued at USD 0.89 Billion in 2023, and is Projected to Reach USD 1.56 Billion by 2032, Growing at a CAGR of 6.43% From 2024-2032.
C difficile is a spore-forming gram-positive anaerobic bacterium which can cause severe gastrointestinal infection on the background of antibiotic treatment mainly colonisation with diarrhoea and colitis. The market for C. diagnostics and treatment involves products such as diagnostic tools for testing or identifying CDI and those products intended for treatment of the same. From the diagnostic technique, PCR, EIA, and culture methods are some of the techniques used while the treatment methods are; antibiotics such as vancomycin and fidaxomicin, faecal microbiota transplantation (FMT). The need for a product that can fight C. difficile infection has grown over the years due to the rise of the infection incidence rates around the world, with increased hospital-acquired infection rates and worrisome new strains of the bug.
Due to increase in fear that C. difficile symptoms are frequent and severe, there is increased global market on diagnostics and treatment of Clostridium Difficile. CDI, and particularly its antecedent, has recently risen especially in hospitalized individuals and those taking long-term antibiotics, which in turn has raised the demand for reliable markers and efficient regimens. C. difficile has emerged as a critical healthcare issue due to new emerging strains such as ribotype 027 which are more virulent, resistant to antibiotics and will more likely cause a relapse. CDI burden is also experienced because of the increased elderly patient population and more patients with underlying diseases like cancer or diabetes.
In terms of diagnostics, the market is mainly based on the increased use of newly developed molecular approaches including PCR and NGS in contrast with conventional catalytic techniques. Also, constant diagnostics are the rapidly increasing uses of rapid diagnostic tests (RDTs) of C. difficile due to its ease and speediness in diagnosing clinical case of infection to enhance reduced spreading of the infection.

Clostridium Difficile Diagnostics and Treatment Market Trend Analysis:
Shift Towards Molecular Diagnostics and Rapid Tests
- A clear indication in the global C. difficile diagnostics market is that its market is shifting towards molecular diagnostics using PCR and NGS. These options are emerging due to increased sensitivity and specificity over conventional detection procedures such as EIA and stool samples. The molecular identify of this pathogen can detect the toxin or the gene associated with C. difficile with higher sensitivity and specificity compared to traditional methods, which translates to better decisions made by clinicians. This shift is important as it can lead to prevention of spread of the infection because the problem is addressed at its early stages before it goes viral in places such as hospitals and care homes where infections are most likely to happen.
- Other methods such as PCR, NGS and the more recently favoured rapid diagnostic tests (RDTs). These tests give a first result within hours, thus cutting down a lot of time that patients spend in waiting for a diagnosis and subsequent treatment. It is important for the rapid diagnostics to be accurate and fast because delay in identifying C. difficile is detrimental to its management since patients can easily spread the bacteria to other individuals and certainly more damaging complications ensue. Given the increasing global focus on accurate and quick diagnosis, molecular and rapid diagnostic tests shall also be the major driving forces in the C. difficile diagnostics market to contain their utility in clinical practices.
Growing Demand for Innovative Treatment Options
- There is a big gap in the market concerning C. Diff treatment; this is because there is increased occurrence of recurrent C. Diff infections and antibiotic resistance. Although the conventional treatments include vancomycin, and fidaxomicin, a significant number of patients experience recurrent infection and hence prolonged hospitalization, high healthcare costs. This leads to high demand for other therapies with the capacity to cure the infection as well as prevent the reoccurrence. A realistic solution is faecal microbiota transplantation (FMT), which has lately attracted interest. This form of effective works by re-establishing the normal composition of gut microbiota and may be less likely to lead to further CDI infections so is likely to be a better option for patients who are experiencing repeat infections.
- Moreover, increased concern regarding the increased antibiotic resistance in C. difficile strains have made the researchers look for suitable antibiotic and non-antibiotic treatment. Newer forms of antitoxin therapies are monoclonal antibodies and biologics that work more specifically in the form of toxins developed by C. difficile. As the concept of ‘personalised medicine’ grows, so does the idea of the microbes living inside each patient’s body, and their state of overall health or disease. This increasing awareness of the gut microbiome and its potential in managing infections is a golden opportunity for pharmaceutical organizations to introduce new and efficient remedies that can fill these gaps and get a better result for C. difficile patients to help alleviate the recurrent infections burden.
Clostridium Difficile Diagnostics and Treatment Market Segment Analysis:
Clostridium Difficile Diagnostics and Treatment Market is Segmented on the basis of Diagnostics, Treatment, End User, and Region
By Diagnostics, stool culture segment is expected to dominate the market during the forecast period
- Molecular diagnostics is on the rise and PCR and the more complex NGS are today the market standard in the diagnostics segment of Clostridium Difficile. These techniques have improved the sensitivity and specificity and provide quick results that make the identification of C. difficile more prompt among different health care givers. PCR based methods and NGS methods detect toxin or genetic material of C. difficile, thus avoiding false positive or false negatives which are important in controlling healthcare-associated outbreaks. Owing to the increase in antibiotic resistance and recurrent infections, molecular diagnostics have recently received good support in hospital and outpatient care settings.
- Other diagnostic methods include immunoassays e.g. The enzyme immunoassays (EIA) which have been in common use in clinical laboratories. Although ELISA tests are not as sensitive as molecular diagnostic, they remain popular because of their lower cost and shorter turnaround time. Stool culture is another method, still important, but is not rapid and economic as molecular methods. Other supplementary diagnostic procedures, including imaging and biomolecular analyses, are currently still in the experimental stage but present potentialities for further diversification of diagnostic approaches in C. difficile associated infections. Since the users require faster and more accurate results, such methods are likely to develop further.
By Treatment, Probiotics segment expected to held the largest share
- In the fight against CDI, antibiotics are the key weapon to date. Current treatment for CDI with antibiotics includes vancomycin, fidaxomicin, and metronidazole. whereas fidaxomicin has the potential of reducing recurrences This antibiotic is usually reserved for severe cases. While they are effective, metronidazole can have side effects when taken daily or for a long time – that is why it is best used for mild cases of infections. Nonetheless, many patients have repeated infections even with antibiotic treatments, and hence there are other form of treatment. These antibiotics help to control the infection though the emerging problem of antibiotic resistance call for better and specific treatments.
- FMT has been identified as a very effective treatment for recurrent CDI. FMT entails the implantation of faecal matter from a donor to the patient with an aim of replacing correct gut bacteria that help to counter future infections due to C. difficile alteration of the patients’ digestion system. Other developing therapies include monoclonal antibodies including bezlotoxumab. These antibodies selectively bind and inactivate C. difficile toxins thus allowing the prevention of a recurrence. Probiotics while not as frequently utilized are being studied to supplement other treatments to help rehabilitate the bowel and avert relapse. Other treatment currently under research includes bacteriophage therapy and vaccines, which could provide cure to eradicade C. difficile.
Clostridium Difficile Diagnostics and Treatment Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
- North America and particularly United States retained the largest share in the Clostridium difficile diagnostics and treatment market in 2023. This is due to the high incidence of healthcare associated infection (HAIs) in the region and hence the focus on infection prevention and efficient diagnostics and therapeutic path-ways. C. difficile infections come second after the primary nosocomial gastrointestinal infections in the United States, for a higher need for a better diagnostic measure and appropriate treatment. The market for C. difficile diagnostics and treatment was estimated that the United States represents more than 40% of the global market in 2023, which proves that the area is very important for the global market.
- This advantage is further supplemented by the robust international health care market that provides versatile access to rapidly integrate and develop hi-tech diagnostic tools such as molecular diagnostics, and rapid test kits. These innovations play an important role in increasing the efficiency of C. difficile diagnosis – especially in hospitals, where the bacteria acts as a main infection source. Also, it has well equipped major players in pharmaceutical and medical devices industries who are vigorously involved in the research and development of better therapies including faecal microbiota transplants (FMT) and monoclonal antibodies in North America. All these factors have provided synergy and elevated North America as the leading market for C. difficile diagnostics and treatment globally.
Active Key Players in the Clostridium Difficile Diagnostics and Treatment Market:
- Abbott Laboratories (USA)
- Astellas Pharma Inc. (Japan)
- Biomerieux SA (France)
- Biomérieux, Inc. (France)
- Cosmo Pharmaceuticals N.V. (Ireland)
- Cubist Pharmaceuticals, Inc. (USA)
- Ferring Pharmaceuticals (Switzerland)
- Merck & Co., Inc. (USA)
- Pfizer Inc. (USA)
- Roche Diagnostics (Switzerland)
- Sandoz (Germany)
- Seres Therapeutics (USA)
- Summit Therapeutics (USA)
- Takeda Pharmaceuticals (Japan)
- Zambon S.p.A. (Italy), and Other Active Players.
|
Global Clostridium Difficile Diagnostics and Treatment Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 0.89 Billion |
|
Forecast Period 2024-32 CAGR: |
6.43 % |
Market Size in 2032: |
USD 1.56 Billion |
|
Segments Covered: |
By Diagnostics |
|
|
|
By Treatment |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Clostridium Difficile Diagnostics and Treatment Market by By Diagnostics (2018-2032)
4.1 Clostridium Difficile Diagnostics and Treatment Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Molecular Diagnostics (e.g.
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 PCR
4.5 Next-Generation Sequencing)
4.6 Immunoassays (e.g.
4.7 Enzyme Immunoassays)
4.8 Stool Culture
4.9 Other Diagnostic Methods
Chapter 5: Clostridium Difficile Diagnostics and Treatment Market by By Treatment (2018-2032)
5.1 Clostridium Difficile Diagnostics and Treatment Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Antibiotics (e.g.
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Vancomycin
5.5 Fidaxomicin
5.6 Metronidazole)
5.7 Faecal Microbiota Transplantation (FMT)
5.8 Monoclonal Antibodies (e.g.
5.9 Bezlotoxumab)
5.10 Probiotics
5.11 Other Treatment Methods
Chapter 6: Clostridium Difficile Diagnostics and Treatment Market by By End User (2018-2032)
6.1 Clostridium Difficile Diagnostics and Treatment Market Snapshot and Growth Engine
6.2 Market Overview
6.3 Hospitals
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
6.3.3 Key Market Trends, Growth Factors, and Opportunities
6.3.4 Geographic Segmentation Analysis
6.4 Clinics
6.5 Long-Term Care Facilities
6.6 Outpatient Facilities
6.7 Others
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 Clostridium Difficile Diagnostics and Treatment Market Share by Manufacturer (2024)
7.1.3 Industry BCG Matrix
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 ABBOTT LABORATORIES (USA)
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Key Strategic Moves and Recent Developments
7.2.10 SWOT Analysis
7.3 ASTELLAS PHARMA INC. (JAPAN)
7.4 BIOMERIEUX SA (FRANCE)
7.5 BIOMERIEUX INC. (FRANCE)
7.6 COSMO PHARMACEUTICALS N.V. (IRELAND)
7.7 OTHER ACTIVE PLAYERS
Chapter 8: Global Clostridium Difficile Diagnostics and Treatment Market By Region
8.1 Overview
8.2. North America Clostridium Difficile Diagnostics and Treatment Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecasted Market Size By By Diagnostics
8.2.4.1 Molecular Diagnostics (e.g.
8.2.4.2 PCR
8.2.4.3 Next-Generation Sequencing)
8.2.4.4 Immunoassays (e.g.
8.2.4.5 Enzyme Immunoassays)
8.2.4.6 Stool Culture
8.2.4.7 Other Diagnostic Methods
8.2.5 Historic and Forecasted Market Size By By Treatment
8.2.5.1 Antibiotics (e.g.
8.2.5.2 Vancomycin
8.2.5.3 Fidaxomicin
8.2.5.4 Metronidazole)
8.2.5.5 Faecal Microbiota Transplantation (FMT)
8.2.5.6 Monoclonal Antibodies (e.g.
8.2.5.7 Bezlotoxumab)
8.2.5.8 Probiotics
8.2.5.9 Other Treatment Methods
8.2.6 Historic and Forecasted Market Size By By End User
8.2.6.1 Hospitals
8.2.6.2 Clinics
8.2.6.3 Long-Term Care Facilities
8.2.6.4 Outpatient Facilities
8.2.6.5 Others
8.2.7 Historic and Forecast Market Size by Country
8.2.7.1 US
8.2.7.2 Canada
8.2.7.3 Mexico
8.3. Eastern Europe Clostridium Difficile Diagnostics and Treatment Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecasted Market Size By By Diagnostics
8.3.4.1 Molecular Diagnostics (e.g.
8.3.4.2 PCR
8.3.4.3 Next-Generation Sequencing)
8.3.4.4 Immunoassays (e.g.
8.3.4.5 Enzyme Immunoassays)
8.3.4.6 Stool Culture
8.3.4.7 Other Diagnostic Methods
8.3.5 Historic and Forecasted Market Size By By Treatment
8.3.5.1 Antibiotics (e.g.
8.3.5.2 Vancomycin
8.3.5.3 Fidaxomicin
8.3.5.4 Metronidazole)
8.3.5.5 Faecal Microbiota Transplantation (FMT)
8.3.5.6 Monoclonal Antibodies (e.g.
8.3.5.7 Bezlotoxumab)
8.3.5.8 Probiotics
8.3.5.9 Other Treatment Methods
8.3.6 Historic and Forecasted Market Size By By End User
8.3.6.1 Hospitals
8.3.6.2 Clinics
8.3.6.3 Long-Term Care Facilities
8.3.6.4 Outpatient Facilities
8.3.6.5 Others
8.3.7 Historic and Forecast Market Size by Country
8.3.7.1 Russia
8.3.7.2 Bulgaria
8.3.7.3 The Czech Republic
8.3.7.4 Hungary
8.3.7.5 Poland
8.3.7.6 Romania
8.3.7.7 Rest of Eastern Europe
8.4. Western Europe Clostridium Difficile Diagnostics and Treatment Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecasted Market Size By By Diagnostics
8.4.4.1 Molecular Diagnostics (e.g.
8.4.4.2 PCR
8.4.4.3 Next-Generation Sequencing)
8.4.4.4 Immunoassays (e.g.
8.4.4.5 Enzyme Immunoassays)
8.4.4.6 Stool Culture
8.4.4.7 Other Diagnostic Methods
8.4.5 Historic and Forecasted Market Size By By Treatment
8.4.5.1 Antibiotics (e.g.
8.4.5.2 Vancomycin
8.4.5.3 Fidaxomicin
8.4.5.4 Metronidazole)
8.4.5.5 Faecal Microbiota Transplantation (FMT)
8.4.5.6 Monoclonal Antibodies (e.g.
8.4.5.7 Bezlotoxumab)
8.4.5.8 Probiotics
8.4.5.9 Other Treatment Methods
8.4.6 Historic and Forecasted Market Size By By End User
8.4.6.1 Hospitals
8.4.6.2 Clinics
8.4.6.3 Long-Term Care Facilities
8.4.6.4 Outpatient Facilities
8.4.6.5 Others
8.4.7 Historic and Forecast Market Size by Country
8.4.7.1 Germany
8.4.7.2 UK
8.4.7.3 France
8.4.7.4 The Netherlands
8.4.7.5 Italy
8.4.7.6 Spain
8.4.7.7 Rest of Western Europe
8.5. Asia Pacific Clostridium Difficile Diagnostics and Treatment Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecasted Market Size By By Diagnostics
8.5.4.1 Molecular Diagnostics (e.g.
8.5.4.2 PCR
8.5.4.3 Next-Generation Sequencing)
8.5.4.4 Immunoassays (e.g.
8.5.4.5 Enzyme Immunoassays)
8.5.4.6 Stool Culture
8.5.4.7 Other Diagnostic Methods
8.5.5 Historic and Forecasted Market Size By By Treatment
8.5.5.1 Antibiotics (e.g.
8.5.5.2 Vancomycin
8.5.5.3 Fidaxomicin
8.5.5.4 Metronidazole)
8.5.5.5 Faecal Microbiota Transplantation (FMT)
8.5.5.6 Monoclonal Antibodies (e.g.
8.5.5.7 Bezlotoxumab)
8.5.5.8 Probiotics
8.5.5.9 Other Treatment Methods
8.5.6 Historic and Forecasted Market Size By By End User
8.5.6.1 Hospitals
8.5.6.2 Clinics
8.5.6.3 Long-Term Care Facilities
8.5.6.4 Outpatient Facilities
8.5.6.5 Others
8.5.7 Historic and Forecast Market Size by Country
8.5.7.1 China
8.5.7.2 India
8.5.7.3 Japan
8.5.7.4 South Korea
8.5.7.5 Malaysia
8.5.7.6 Thailand
8.5.7.7 Vietnam
8.5.7.8 The Philippines
8.5.7.9 Australia
8.5.7.10 New Zealand
8.5.7.11 Rest of APAC
8.6. Middle East & Africa Clostridium Difficile Diagnostics and Treatment Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecasted Market Size By By Diagnostics
8.6.4.1 Molecular Diagnostics (e.g.
8.6.4.2 PCR
8.6.4.3 Next-Generation Sequencing)
8.6.4.4 Immunoassays (e.g.
8.6.4.5 Enzyme Immunoassays)
8.6.4.6 Stool Culture
8.6.4.7 Other Diagnostic Methods
8.6.5 Historic and Forecasted Market Size By By Treatment
8.6.5.1 Antibiotics (e.g.
8.6.5.2 Vancomycin
8.6.5.3 Fidaxomicin
8.6.5.4 Metronidazole)
8.6.5.5 Faecal Microbiota Transplantation (FMT)
8.6.5.6 Monoclonal Antibodies (e.g.
8.6.5.7 Bezlotoxumab)
8.6.5.8 Probiotics
8.6.5.9 Other Treatment Methods
8.6.6 Historic and Forecasted Market Size By By End User
8.6.6.1 Hospitals
8.6.6.2 Clinics
8.6.6.3 Long-Term Care Facilities
8.6.6.4 Outpatient Facilities
8.6.6.5 Others
8.6.7 Historic and Forecast Market Size by Country
8.6.7.1 Turkiye
8.6.7.2 Bahrain
8.6.7.3 Kuwait
8.6.7.4 Saudi Arabia
8.6.7.5 Qatar
8.6.7.6 UAE
8.6.7.7 Israel
8.6.7.8 South Africa
8.7. South America Clostridium Difficile Diagnostics and Treatment Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecasted Market Size By By Diagnostics
8.7.4.1 Molecular Diagnostics (e.g.
8.7.4.2 PCR
8.7.4.3 Next-Generation Sequencing)
8.7.4.4 Immunoassays (e.g.
8.7.4.5 Enzyme Immunoassays)
8.7.4.6 Stool Culture
8.7.4.7 Other Diagnostic Methods
8.7.5 Historic and Forecasted Market Size By By Treatment
8.7.5.1 Antibiotics (e.g.
8.7.5.2 Vancomycin
8.7.5.3 Fidaxomicin
8.7.5.4 Metronidazole)
8.7.5.5 Faecal Microbiota Transplantation (FMT)
8.7.5.6 Monoclonal Antibodies (e.g.
8.7.5.7 Bezlotoxumab)
8.7.5.8 Probiotics
8.7.5.9 Other Treatment Methods
8.7.6 Historic and Forecasted Market Size By By End User
8.7.6.1 Hospitals
8.7.6.2 Clinics
8.7.6.3 Long-Term Care Facilities
8.7.6.4 Outpatient Facilities
8.7.6.5 Others
8.7.7 Historic and Forecast Market Size by Country
8.7.7.1 Brazil
8.7.7.2 Argentina
8.7.7.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
|
Global Clostridium Difficile Diagnostics and Treatment Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 0.89 Billion |
|
Forecast Period 2024-32 CAGR: |
6.43 % |
Market Size in 2032: |
USD 1.56 Billion |
|
Segments Covered: |
By Diagnostics |
|
|
|
By Treatment |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Frequently Asked Questions :
The forecast period in the Clostridium Difficile Diagnostics and Treatment Market research report is 2024-2032.
Abbott Laboratories (USA), Astellas Pharma Inc. (Japan), Biomerieux SA (France), Biomérieux, Inc. (France), Cosmo Pharmaceuticals N.V. (Ireland), Cubist Pharmaceuticals, Inc. (USA), Ferring Pharmaceuticals (Switzerland), Merck & Co., Inc. (USA), Pfizer Inc. (USA), Roche Diagnostics (Switzerland), Sandoz (Germany), Seres Therapeutics (USA), Summit Therapeutics (USA), Takeda Pharmaceuticals (Japan), Zambon S.p.A. (Italy), and Other Active Players.
The Clostridium Difficile Diagnostics and Treatment Market is segmented into Type, Application, End User and region. By Diagnostics, the market is categorized into Molecular Diagnostics (e.g., PCR, Next-Generation Sequencing), Immunoassays (e.g., Enzyme Immunoassays), Stool Culture, Other Diagnostic Methods. By Treatment, the market is categorized into Antibiotics (e.g., Vancomycin, Fidaxomicin, Metronidazole), Faecal Microbiota Transplantation (FMT), Monoclonal Antibodies (e.g., Bezlotoxumab), Probiotics, Other Treatment Methods. By End User, the market is categorized into Hospitals, Clinics, Long-Term Care Facilities, Outpatient Facilities, Others. By region, it is analyzed across North America (U.S., Canada, Mexico), Eastern Europe (Russia, Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe), Western Europe (Germany, UK, France, The Netherlands, Italy, Spain, Rest of Western Europe), Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New-Zealand, Rest of APAC), Middle East & Africa (Turkiye, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa), South America (Brazil, Argentina, Rest of SA).
C difficile is a spore-forming gram-positive anaerobic bacterium which can cause severe gastrointestinal infection on the background of antibiotic treatment mainly colonization with diarrhea and colitis. The market for C. diagnostics and treatment involves products such as diagnostic tools for testing or identifying CDI and those products intended for treatment of the same. From the diagnostic technique, PCR, EIA, and culture methods are some of the techniques used while the treatment methods are; antibiotics such as vancomycin and fidaxomicin, faecal microbiota transplantation (FMT). The need for a product that can fight C. difficile infection has grown over the years due to the rise of the infection incidence rates around the world, with increased hospital-acquired infection rates and worrisome new strains of the bug.
Clostridium Difficile Diagnostics and Treatment Market Size Was Valued at USD 0.89 Billion in 2023, and is Projected to Reach USD 1.56 Billion by 2032, Growing at a CAGR of 6.43% From 2024-2032.








