Gene Therapy for Rare Disease Market Synopsis:
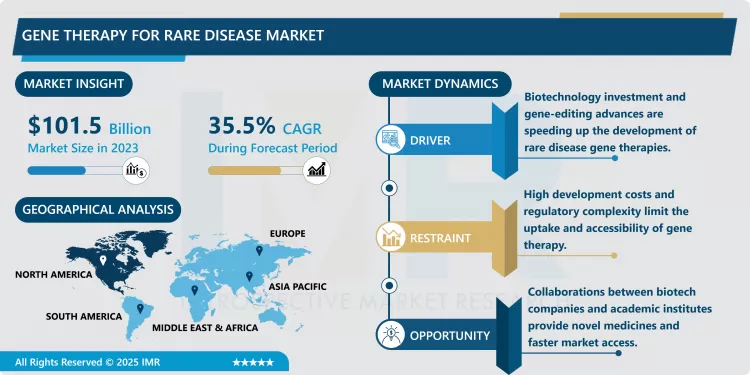
Gene Therapy for Rare Disease Market Size Was Valued at USD 101.5 Billion in 2023, and is Projected to Reach USD 2,869.54 Billion by 2032, Growing at a CAGR of 35.5% From 2024-2032.
The Gene Therapy for Rare Disease Market has come on strong as developments in gene therapy have made it possible to tailor methods in a better way. Gene therapies being the novel and experimental treatment to cure diseases with fewer numbers of victims, rare diseases have started to emerge as the subsequent candidate for breakthroughs. This market has been supported by enhanced knowledge on rare diseases, government support for orphan drug development in addition to innovations in gene modification technologies like CRISPR. Such developments help to imagine one shot cure for patients, in terms of both, quality of life and longer life expectancy.
There has been increase funding in gene therapy sciences through public and private investors.. Modern pharma and Biotech companies are investing considerable efforts to discover therapies for genetic diseases that include DMD and SMA as well as hemophilia among others. Using approval guidelines by agencies such as the FDA and EMA, the market for gene therapies targeting rare disease has been given approval to fast track its growth. University and research institute-industry interactions are ever increasing as part of medical innovation and faster clinical trial activities.• However, the Gene Therapy for Rare Disease Market has several challenges as follows;.or funding. Pharmaceutical companies and biotech firms are making significant strides in developing therapies for genetic disorders, including Duchenne muscular dystrophy, spinal muscular atrophy, and hemophilia, among others. Regulatory bodies like the FDA and EMA have expedited approval processes for gene therapies addressing rare diseases, which accelerates the market’s growth. Collaborations between universities, research institutes, and pharmaceutical companies are also on the rise, fostering an ecosystem conducive to innovation and quicker clinical trials.
Despite its promise, the Gene Therapy for Rare Disease Market faces several challenges. This means that these therapies are expensive to administer due to their high cost of treatments, involved processes of production, and policies involving health care products and services may also limit the use of these therapies. Also limitations such as the small pools of patient population challenges the clinical trials as getting enough participants to test the efficacy is Tasking. On the contrary, recent innovations in vector construction and manufacturing, coupled with pressures for cost effective solutions suggest the gene therapy market for rare diseases is viable and will become robust in the future as more effective, cheaper solutions are developed.

Gene Therapy for Rare Disease Market Trend Analysis:
Increasing Investment and Partnerships in Gene Therapy Development
- The shifting towards regenerative medicine is one of the most prominent trends regarding the Gene Therapy for Rare Disease Market and particular attention to gene therapy as a potentially lucrative industry is another one. A number of big pharma’s, biotech firms and venture capitalists are investing huge bucks in gene therapy programs with special emphasis on diseases with no cure or inadequate treatment. Existing partnerships between players in the industry and research institutions are also broadening to share knowledge, capital, and information. This trend is promoting the speed of clinical trials, as several companies are partnering up to deal with high R&D expenses, complicated regulators, and technologic issues in production. Hence the pipeline of Gene Therapies for Rare Diseases is reasonably well-developed and more products from the shelf are expected in the future meeting the needs of one or the other patient population.
Advancements in Gene-Editing Technology and Delivery Mechanisms
- A second important factor in the Gene Therapy for Rare Disease Market involves the continued progress of gene editing technology, especially CRISPR, and enhancements to delivery systems.. Current advancements of CRISPR technology make it possible to target and correct problem-genes and therefore tackle the origins of the genetic disorders. Parallel to these progresses, there are progresses in the delivery systems; viral vectors and nanoparticle based systems, which improve the efficiency and specificity of gene therapies. Such advances the improving the gene-editing technology and delivery systems reducing the likelihood of side effects while enhancing the effectiveness of the therapy makes gene therapy a revolutionary treatment for patients with such neurological disorders.
Gene Therapy for Rare Disease Market Segment Analysis:
Gene Therapy for Rare Disease Market is Segmented on the basis of Source, application, And Region
By Drug, Approved Drugs segment is expected to dominate the market during the forecast period
- The Gene Therapy for Rare Disease Market has advanced significantly with the approval of some incredible drugs pointing to genetic diseases.. These are services are Tisagenlecleucel (Kymriah) and Axicabtagene ciloleucel (Yescarta) that are CAR T-cell therapies intended mainly for various forms of blood cancer and Voretigene neparvovec (Luxturna) for inherited retinal diseases. Also, the company has come up with Strimvelis, a gene therapy that has received approval for treating severe combined immunodeficiency (SCID) patients as their options are limited to therapy. These drugs have been identified as major breakthroughs as they show what gene therapy can offer to the market moving from untreatable or lethal diseases to a wider use in the rare diseases domain.
By Therapeutic Application, Neurological Disorders segment expected to held the largest share
- The Gene Therapy for Rare Disease Market covers several disease areas of therapeutic intervention to address unmet medical needs such as oncologic, neurologic, ophthalmologic, hematologic, immunologic, metabolic and other diseases.. Gene therapy is slowly finding its way into oncology especially targeting rare type cancers that have no cure. Spinal muscular atrophy is one of the neurodegenerative diseases; gene therapy provides an opportunity for one-time correction of nerve damage. Also in ophthalmology, gene therapies of inherited retinal diseases including Leber congenital amaurosis are showing better scores in visual acuity for patients. Many haematological diseases, including haemophilia, are experiencing an emergent therapy that Gene therapy can provide long term or curative advantage. However, immunodeficiency and metabolic disorders where gene therapy has emerged to try to correct the genetic dysfunctions, there is hope for patients who in the past had few treatment options. As these technologies have advanced across these therapeutic applications, the gene therapy market for rare diseases will likely expand further where more therapies receive regulation and clinical use.
Gene Therapy for Rare Disease Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
- North America is expected to have the largest share in the Gene Therapy for Rare Disease Market for the duration of the prognosis because of factors such as extensive funds spending, improved research facilities, and favorable governmental policies.. In particular, the United States takes a leading position in this segment due to the concentration of a large number of biotechnology companies, well-equipped clinical trials centers, and increased government investment in the field of rare disease study. Further, the authorities such as the FDA have established fast-track and orphan drug status to increase the subjugation of the approval procedures harboring gene therapies for rare diseases. Having such a supportive culture alongside growing refined awareness of low-frequency genetic diseases as well as the general use of gene editing technologies puts North America as one of the excellent regions for development in the gene therapy market.
Active Key Players in the Gene Therapy for Rare Disease Market:
- Kite Pharma, Inc. (Gilead Sciences, Inc.) (USA)
- Novartis International AG (Switzerland)
- Juno Therapeutics Inc. (Celgene Corporation) (USA)
- Bluebird Bio, Inc. (USA)
- Spark Therapeutics, Inc. (USA)
- uniQure N.V (Netherlands)
- Orchard Therapeutics Plc. (United Kingdom)
- PTC Therapeutics, Inc. (USA)
- BioMarin Pharmaceutical Inc. (USA)
- Other Active Players
|
Gene Therapy for Rare Disease Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 101.5 Billion |
|
Forecast Period 2024-32 CAGR: |
35.5% |
Market Size in 2032: |
USD 1,562.91 Billion |
|
Segments Covered: |
By Drug |
|
|
|
Pipeline Drugs |
|
||
|
By Therapeutic Application |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Gene Therapy for Rare Disease Market by By Drug (2018-2032)
4.1 Gene Therapy for Rare Disease Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Approved Drugs (Tisagenlecleucel (Kymriah)
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 Axicabtagene ciloleucel (Yescarta)
4.5 Voretigene neparvovec (Luxturna)
4.6 Strimvelis
4.7 Pipeline Drugs
4.8 GTAADC
4.9 Fidanacogene elaparvovec (SPK-9011)
4.10 OTL-200
4.11 bb2121
4.12 AMT-061
4.13 Others
Chapter 5: Gene Therapy for Rare Disease Market by By Therapeutic Application (2018-2032)
5.1 Gene Therapy for Rare Disease Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Oncology
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Neurological Disorders
5.5 Ophthalmic Disorders
5.6 Hematological Disorders
5.7 Immunodeficiency Disorders
5.8 Metabolic Disorders
5.9 Others
Chapter 6: Company Profiles and Competitive Analysis
6.1 Competitive Landscape
6.1.1 Competitive Benchmarking
6.1.2 Gene Therapy for Rare Disease Market Share by Manufacturer (2024)
6.1.3 Industry BCG Matrix
6.1.4 Heat Map Analysis
6.1.5 Mergers and Acquisitions
6.2 KITE PHARMA INC. (GILEAD SCIENCES INC.) (USA)
6.2.1 Company Overview
6.2.2 Key Executives
6.2.3 Company Snapshot
6.2.4 Role of the Company in the Market
6.2.5 Sustainability and Social Responsibility
6.2.6 Operating Business Segments
6.2.7 Product Portfolio
6.2.8 Business Performance
6.2.9 Key Strategic Moves and Recent Developments
6.2.10 SWOT Analysis
6.3 NOVARTIS INTERNATIONAL AG (SWITZERLAND)
6.4 JUNO THERAPEUTICS INC. (CELGENE CORPORATION) (USA)
6.5 BLUEBIRD BIO INC. (USA)
6.6 SPARK THERAPEUTICS INC. (USA)
6.7 UNIQURE N.V (NETHERLANDS)
6.8 ORCHARD THERAPEUTICS PLC. (UNITED KINGDOM)
6.9 PTC THERAPEUTICS INC. (USA)
6.10 BIOMARIN PHARMACEUTICAL INC. (USA)
6.11 OTHER ACTIVE PLAYERS
Chapter 7: Global Gene Therapy for Rare Disease Market By Region
7.1 Overview
7.2. North America Gene Therapy for Rare Disease Market
7.2.1 Key Market Trends, Growth Factors and Opportunities
7.2.2 Top Key Companies
7.2.3 Historic and Forecasted Market Size by Segments
7.2.4 Historic and Forecasted Market Size By By Drug
7.2.4.1 Approved Drugs (Tisagenlecleucel (Kymriah)
7.2.4.2 Axicabtagene ciloleucel (Yescarta)
7.2.4.3 Voretigene neparvovec (Luxturna)
7.2.4.4 Strimvelis
7.2.4.5 Pipeline Drugs
7.2.4.6 GTAADC
7.2.4.7 Fidanacogene elaparvovec (SPK-9011)
7.2.4.8 OTL-200
7.2.4.9 bb2121
7.2.4.10 AMT-061
7.2.4.11 Others
7.2.5 Historic and Forecasted Market Size By By Therapeutic Application
7.2.5.1 Oncology
7.2.5.2 Neurological Disorders
7.2.5.3 Ophthalmic Disorders
7.2.5.4 Hematological Disorders
7.2.5.5 Immunodeficiency Disorders
7.2.5.6 Metabolic Disorders
7.2.5.7 Others
7.2.6 Historic and Forecast Market Size by Country
7.2.6.1 US
7.2.6.2 Canada
7.2.6.3 Mexico
7.3. Eastern Europe Gene Therapy for Rare Disease Market
7.3.1 Key Market Trends, Growth Factors and Opportunities
7.3.2 Top Key Companies
7.3.3 Historic and Forecasted Market Size by Segments
7.3.4 Historic and Forecasted Market Size By By Drug
7.3.4.1 Approved Drugs (Tisagenlecleucel (Kymriah)
7.3.4.2 Axicabtagene ciloleucel (Yescarta)
7.3.4.3 Voretigene neparvovec (Luxturna)
7.3.4.4 Strimvelis
7.3.4.5 Pipeline Drugs
7.3.4.6 GTAADC
7.3.4.7 Fidanacogene elaparvovec (SPK-9011)
7.3.4.8 OTL-200
7.3.4.9 bb2121
7.3.4.10 AMT-061
7.3.4.11 Others
7.3.5 Historic and Forecasted Market Size By By Therapeutic Application
7.3.5.1 Oncology
7.3.5.2 Neurological Disorders
7.3.5.3 Ophthalmic Disorders
7.3.5.4 Hematological Disorders
7.3.5.5 Immunodeficiency Disorders
7.3.5.6 Metabolic Disorders
7.3.5.7 Others
7.3.6 Historic and Forecast Market Size by Country
7.3.6.1 Russia
7.3.6.2 Bulgaria
7.3.6.3 The Czech Republic
7.3.6.4 Hungary
7.3.6.5 Poland
7.3.6.6 Romania
7.3.6.7 Rest of Eastern Europe
7.4. Western Europe Gene Therapy for Rare Disease Market
7.4.1 Key Market Trends, Growth Factors and Opportunities
7.4.2 Top Key Companies
7.4.3 Historic and Forecasted Market Size by Segments
7.4.4 Historic and Forecasted Market Size By By Drug
7.4.4.1 Approved Drugs (Tisagenlecleucel (Kymriah)
7.4.4.2 Axicabtagene ciloleucel (Yescarta)
7.4.4.3 Voretigene neparvovec (Luxturna)
7.4.4.4 Strimvelis
7.4.4.5 Pipeline Drugs
7.4.4.6 GTAADC
7.4.4.7 Fidanacogene elaparvovec (SPK-9011)
7.4.4.8 OTL-200
7.4.4.9 bb2121
7.4.4.10 AMT-061
7.4.4.11 Others
7.4.5 Historic and Forecasted Market Size By By Therapeutic Application
7.4.5.1 Oncology
7.4.5.2 Neurological Disorders
7.4.5.3 Ophthalmic Disorders
7.4.5.4 Hematological Disorders
7.4.5.5 Immunodeficiency Disorders
7.4.5.6 Metabolic Disorders
7.4.5.7 Others
7.4.6 Historic and Forecast Market Size by Country
7.4.6.1 Germany
7.4.6.2 UK
7.4.6.3 France
7.4.6.4 The Netherlands
7.4.6.5 Italy
7.4.6.6 Spain
7.4.6.7 Rest of Western Europe
7.5. Asia Pacific Gene Therapy for Rare Disease Market
7.5.1 Key Market Trends, Growth Factors and Opportunities
7.5.2 Top Key Companies
7.5.3 Historic and Forecasted Market Size by Segments
7.5.4 Historic and Forecasted Market Size By By Drug
7.5.4.1 Approved Drugs (Tisagenlecleucel (Kymriah)
7.5.4.2 Axicabtagene ciloleucel (Yescarta)
7.5.4.3 Voretigene neparvovec (Luxturna)
7.5.4.4 Strimvelis
7.5.4.5 Pipeline Drugs
7.5.4.6 GTAADC
7.5.4.7 Fidanacogene elaparvovec (SPK-9011)
7.5.4.8 OTL-200
7.5.4.9 bb2121
7.5.4.10 AMT-061
7.5.4.11 Others
7.5.5 Historic and Forecasted Market Size By By Therapeutic Application
7.5.5.1 Oncology
7.5.5.2 Neurological Disorders
7.5.5.3 Ophthalmic Disorders
7.5.5.4 Hematological Disorders
7.5.5.5 Immunodeficiency Disorders
7.5.5.6 Metabolic Disorders
7.5.5.7 Others
7.5.6 Historic and Forecast Market Size by Country
7.5.6.1 China
7.5.6.2 India
7.5.6.3 Japan
7.5.6.4 South Korea
7.5.6.5 Malaysia
7.5.6.6 Thailand
7.5.6.7 Vietnam
7.5.6.8 The Philippines
7.5.6.9 Australia
7.5.6.10 New Zealand
7.5.6.11 Rest of APAC
7.6. Middle East & Africa Gene Therapy for Rare Disease Market
7.6.1 Key Market Trends, Growth Factors and Opportunities
7.6.2 Top Key Companies
7.6.3 Historic and Forecasted Market Size by Segments
7.6.4 Historic and Forecasted Market Size By By Drug
7.6.4.1 Approved Drugs (Tisagenlecleucel (Kymriah)
7.6.4.2 Axicabtagene ciloleucel (Yescarta)
7.6.4.3 Voretigene neparvovec (Luxturna)
7.6.4.4 Strimvelis
7.6.4.5 Pipeline Drugs
7.6.4.6 GTAADC
7.6.4.7 Fidanacogene elaparvovec (SPK-9011)
7.6.4.8 OTL-200
7.6.4.9 bb2121
7.6.4.10 AMT-061
7.6.4.11 Others
7.6.5 Historic and Forecasted Market Size By By Therapeutic Application
7.6.5.1 Oncology
7.6.5.2 Neurological Disorders
7.6.5.3 Ophthalmic Disorders
7.6.5.4 Hematological Disorders
7.6.5.5 Immunodeficiency Disorders
7.6.5.6 Metabolic Disorders
7.6.5.7 Others
7.6.6 Historic and Forecast Market Size by Country
7.6.6.1 Turkiye
7.6.6.2 Bahrain
7.6.6.3 Kuwait
7.6.6.4 Saudi Arabia
7.6.6.5 Qatar
7.6.6.6 UAE
7.6.6.7 Israel
7.6.6.8 South Africa
7.7. South America Gene Therapy for Rare Disease Market
7.7.1 Key Market Trends, Growth Factors and Opportunities
7.7.2 Top Key Companies
7.7.3 Historic and Forecasted Market Size by Segments
7.7.4 Historic and Forecasted Market Size By By Drug
7.7.4.1 Approved Drugs (Tisagenlecleucel (Kymriah)
7.7.4.2 Axicabtagene ciloleucel (Yescarta)
7.7.4.3 Voretigene neparvovec (Luxturna)
7.7.4.4 Strimvelis
7.7.4.5 Pipeline Drugs
7.7.4.6 GTAADC
7.7.4.7 Fidanacogene elaparvovec (SPK-9011)
7.7.4.8 OTL-200
7.7.4.9 bb2121
7.7.4.10 AMT-061
7.7.4.11 Others
7.7.5 Historic and Forecasted Market Size By By Therapeutic Application
7.7.5.1 Oncology
7.7.5.2 Neurological Disorders
7.7.5.3 Ophthalmic Disorders
7.7.5.4 Hematological Disorders
7.7.5.5 Immunodeficiency Disorders
7.7.5.6 Metabolic Disorders
7.7.5.7 Others
7.7.6 Historic and Forecast Market Size by Country
7.7.6.1 Brazil
7.7.6.2 Argentina
7.7.6.3 Rest of SA
Chapter 8 Analyst Viewpoint and Conclusion
8.1 Recommendations and Concluding Analysis
8.2 Potential Market Strategies
Chapter 9 Research Methodology
9.1 Research Process
9.2 Primary Research
9.3 Secondary Research
|
Gene Therapy for Rare Disease Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 101.5 Billion |
|
Forecast Period 2024-32 CAGR: |
35.5% |
Market Size in 2032: |
USD 1,562.91 Billion |
|
Segments Covered: |
By Drug |
|
|
|
Pipeline Drugs |
|
||
|
By Therapeutic Application |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Frequently Asked Questions :
The forecast period in the Gene Therapy for Rare Disease Market research report is 2024-2032.
Kite Pharma, Inc. (Gilead Sciences, Inc.) (USA), Novartis International AG (Switzerland), Juno Therapeutics Inc. (Celgene Corporation) (USA), Bluebird Bio, Inc. (USA), Spark Therapeutics, Inc. (USA), uniQure N.V (Netherlands), Orchard Therapeutics Plc. (United Kingdom), PTC Therapeutics, Inc. (USA), BioMarin Pharmaceutical Inc. (USA), and Other Active Players.
The Gene Therapy for Rare Disease Market is segmented into By Drug, Pipeline Drugs, By Therapeutic Application and region. By Drug (Approved Drugs (Tisagenlecleucel (Kymriah), Axicabtagene ciloleucel (Yescarta), Voretigene neparvovec (Luxturna), Strimvelis), Pipeline Drugs (GTAADC, Fidanacogene elaparvovec (SPK-9011), OTL-200, bb2121, AMT-061, and Others),By Therapeutic Application (Oncology, Neurological Disorders, Ophthalmic Disorders, Hematological Disorders, Immunodeficiency Disorders, Metabolic Disorders, and Others). By region, it is analyzed across North America (U.S.; Canada; Mexico), Eastern Europe (Bulgaria; The Czech Republic; Hungary; Poland; Romania; Rest of Eastern Europe), Western Europe (Germany; UK; France; Netherlands; Italy; Russia; Spain; Rest of Western Europe), Asia-Pacific (China; India; Japan; Southeast Asia, etc.), South America (Brazil; Argentina, etc.), Middle East & Africa (Saudi Arabia; South Africa, etc.).
The Gene Therapy for Rare Disease Market has come on strong as developments in gene therapy have made it possible to tailor methods in a better way. Gene therapies being the novel and experimental treatment to cure diseases with fewer numbers of victims, rare diseases have started to emerge as the subsequent candidate for breakthroughs. This market has been supported by enhanced knowledge on rare diseases, government support for orphan drug development in addition to innovations in gene modification technologies like CRISPR. Such developments help to imagine one shot cure for patients, in terms of both, quality of life and longer life expectancy.
Gene Therapy for Rare Disease Market Size Was Valued at USD 101.5 Billion in 2023, and is Projected to Reach USD 2,869.54 Billion by 2032, Growing at a CAGR of 35.5% From 2024-2032.








