Osteogenesis Imperfecta Treatment Market Synopsis:
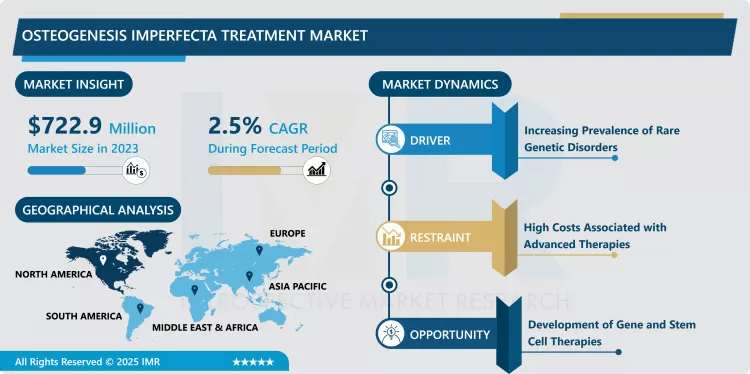
Osteogenesis Imperfecta Treatment Market Size Was Valued at USD 722.9 Million in 2023, and is Projected to Reach USD 902.80 Million by 2032, Growing at a CAGR of 2.5 % From 2024-2032.
The Osteogenesis Imperfecta (OI) Treatment Market includes treatments, medications and therapies employed for easing the conditions of osteogenesis imperfecta, a rare genetic disorder that results in bone fragility, bone fractures and skeletal abnormalities. Management techniques centre on preventing bone loss, preventing bone fractures and promoting the general wellbeing of patients through medication, operations and physiotherapy.
The Osteogenesis Imperfecta Treatment market is growing particularly, thanks to the improvements in the availability and types of treatment available, and a generally higher demand for rare genetic disorders. The molecular and genetic basis of osteogenesis imperfecta is still being recognized, and current and developing treatment methods are bisphosphonates, biologics, and gene therapy. Rising incidence has only added to the list of possibilities, thanks to enhanced diagnostic tool known as genetics.
The increasing availability and concerns towards targeted and specific therapy are beginning to redefine the treatment landscape for this disease, meeting the unmet needs. After several years of research and development, there has been a movement in which drugs needed in the market are specifically disease-modifying, not just merely symptomatic. Further, government legal orders enhancing the availability of therapies for rare diseases and the comparatively higher research project fundage support the growth of this market.

Osteogenesis Imperfecta Treatment Market Trend Analysis:
Rising Adoption of Biologics in Osteogenesis Imperfecta Treatment
- A new trend of the Osteogenesis Imperfecta Treatment Market is the increased use of biologics including Denosumab and monoclonal antibodies. Biologics also have provided substantial improvement in regards to bone mineralization as well as rate of fracture within the severe form of osteogenesis imperfecta. Biologics are more selective compared to old-school bisphosphonates in that they influence the rates of bone remodeling and suppression of osteoclasts. The desire of the heal facilitators to use bio therapeutic products as opposed to others is likely to influence the market in the future due to well-articulated clinical research programs and struggling regulatory enhancements.
Advancements in Gene Therapy for Osteogenesis Imperfecta
- The emergence of gene therapy remains a revolutionary event in the market adjusting for the defective gene that causes osteogenesis imperfecta and replacing it for the improved collagen production. Based on the results obtained in preclinical and phase one and two clinical trials, this approach may greatly transform treatment by delivering sustained outcomes and beneficial impacts on patient health. Future research strategy of the different presses has been acted through collaboration between the biotechnology firms and academic institutions to help speed up the research hence making expansion in this market very lucrative.
Osteogenesis Imperfecta Treatment Market Segment Analysis:
Osteogenesis Imperfecta Treatment Market Segmented on the basis of Drugs, End Route of Administration, and in Region.
By Drugs, Denosumab segment is expected to dominate the market during the forecast period
- The Denosumab segment is expected to dominate the Osteogenesis Imperfecta Treatment Market because it has been shown to increase bone density and decreased the chances of breaks. Denosumab is a RANK ligand inhibitor which due to its mechanisms of action is used in the prevention of bone resorption to improve the general stability of skeletal structure. As a result of its selective action and tolerable side effects profile, it is suitable for the management of moderate to severe forms of osteogenesis imperfecta. Latest developments in clinical research have established Denosumab strictly as a standard therapy for the treatment of this disease. The high rate of use in both children and young adults with asymptomatic and other chronic diseases, along with continual research into LT preserves its position in the market.
By Route of Administration, the Intravenous segment expected to held the largest share
- IV is expected to dominate the osteogenesis imperfecta treatment market, as intravenous bisphosphonates and biologics have gained significant popularity among physicians and patients throughout the world. Iv administration is precise, and it has better bioavailability Compare with other routes, it has better compliance from patients especially those who need strict monitoring. Intravenous pamidronate and Zoledronic acid along with other bisphosphonates are again valid treatments which have been found to lower fracture risks and diminish bone pain. Growth has been supported by increasing severe osteogenesis imperfecta cases and improvements in infusion therapy. The specialized and technological advance healthcare facilities and the distinct infusion centers are expanding their access of these treatments further thus increasing its market.
Osteogenesis Imperfecta Treatment Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
- North America is expected to have the largest market share in the Osteogenesis Imperfecta Treatment Market due to the strong development of the health care industry, increased incidence of rare genetic diseases and the level of outpatient diagnosis. Primarily, the United States holds the lion’s share, due to massive investments in rare diseases and a growth in the clinical trials of innovative products. Another boost to the region’s leadership is the engagement of crucial pharmaceutical stakeholders and legitimate reimbursement policies of orphan drugs.
- Secondly, through the efforts of non-profit organisations and patient self-help groups diagnostic awareness has grown and appropriate treatments have become easier to avail of. Canada is also on the rise as key investor in healthcare as well as increasing concentration on genetic tests and molecular drugs and medicine. However, the studies are being carried out extensively, and North America is also remaining the largest market globally by the forecasted period.
Active Key Players in the Osteogenesis Imperfecta Treatment Market:
- AbbVie Inc. (United States)
- Alexion Pharmaceuticals (United States)
- Amgen Inc. (United States)
- Ascendis Pharma A/S (Denmark)
- Astellas Pharma Inc. (Japan)
- BioMarin Pharmaceutical Inc. (United States)
- Boehringer Ingelheim (Germany)
- Eli Lilly and Company (United States)
- F. Hoffmann-La Roche AG (Switzerland)
- GlaxoSmithKline plc (United Kingdom)
- Johnson & Johnson (United States)
- Merck & Co., Inc. (United States)
- Novartis AG (Switzerland)
- Pfizer Inc. (United States)
- Takeda Pharmaceutical Company Limited (Japan), and Other Active Players.
Key Industry Developments in the Osteogenesis Imperfecta Treatment Market:
- In July 2023, Ultragenyx Pharmaceutical Inc. announced that the first patients were dosed in its Phase 3 clinical trials evaluating setrusumab (UX143) for the treatment of osteogenesis imperfecta (OI). The Phase 3 Orbit study focuses on patients aged 5 to <26 years, assessing setrusumab’s impact on annualized clinical fracture rate compared to placebo. The newly launched Phase 3 Cosmic study evaluates setrusumab against intravenous bisphosphonate therapy in patients aged 2 to <5 years. These trials aim to advance treatment for OI sub-types I, III, and IV, addressing significant unmet medical needs in pediatric and young adult populations.
|
Global Osteogenesis Imperfecta Treatment Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 722.9 Million |
|
Forecast Period 2024-32 CAGR: |
2.5 % |
Market Size in 2032: |
USD 902.80 Million |
|
Segments Covered: |
By Type |
|
|
|
By Route of Administration |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Osteogenesis Imperfecta Treatment Market by By Type (2018-2032)
4.1 Osteogenesis Imperfecta Treatment Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Teriparatide
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 Denosumab
4.5 Others
Chapter 5: Osteogenesis Imperfecta Treatment Market by By Route of Administration (2018-2032)
5.1 Osteogenesis Imperfecta Treatment Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Subcutaneous
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Intravenous
5.5 Oral
5.6 Others
Chapter 6: Company Profiles and Competitive Analysis
6.1 Competitive Landscape
6.1.1 Competitive Benchmarking
6.1.2 Osteogenesis Imperfecta Treatment Market Share by Manufacturer (2024)
6.1.3 Industry BCG Matrix
6.1.4 Heat Map Analysis
6.1.5 Mergers and Acquisitions
6.2 ABBVIE INC. (UNITED STATES)
6.2.1 Company Overview
6.2.2 Key Executives
6.2.3 Company Snapshot
6.2.4 Role of the Company in the Market
6.2.5 Sustainability and Social Responsibility
6.2.6 Operating Business Segments
6.2.7 Product Portfolio
6.2.8 Business Performance
6.2.9 Key Strategic Moves and Recent Developments
6.2.10 SWOT Analysis
6.3 ALEXION PHARMACEUTICALS (UNITED STATES)
6.4 AMGEN INC. (UNITED STATES)
6.5 ASCENDIS PHARMA A/S (DENMARK)
6.6 ASTELLAS PHARMA INC. (JAPAN)
6.7 BIOMARIN PHARMACEUTICAL INC. (UNITED STATES)
6.8 BOEHRINGER INGELHEIM (GERMANY)
6.9 ELI LILLY AND COMPANY (UNITED STATES)
6.10 F. HOFFMANN-LA ROCHE AG (SWITZERLAND)
6.11 GLAXOSMITHKLINE PLC (UNITED KINGDOM)
6.12 JOHNSON & JOHNSON (UNITED STATES)
6.13 MERCK & CO. INC. (UNITED STATES)
6.14 NOVARTIS AG (SWITZERLAND)
6.15 PFIZER INC. (UNITED STATES)
6.16 TAKEDA PHARMACEUTICAL COMPANY LIMITED (JAPAN)
6.17 OTHER ACTIVE PLAYERS
Chapter 7: Global Osteogenesis Imperfecta Treatment Market By Region
7.1 Overview
7.2. North America Osteogenesis Imperfecta Treatment Market
7.2.1 Key Market Trends, Growth Factors and Opportunities
7.2.2 Top Key Companies
7.2.3 Historic and Forecasted Market Size by Segments
7.2.4 Historic and Forecasted Market Size By By Type
7.2.4.1 Teriparatide
7.2.4.2 Denosumab
7.2.4.3 Others
7.2.5 Historic and Forecasted Market Size By By Route of Administration
7.2.5.1 Subcutaneous
7.2.5.2 Intravenous
7.2.5.3 Oral
7.2.5.4 Others
7.2.6 Historic and Forecast Market Size by Country
7.2.6.1 US
7.2.6.2 Canada
7.2.6.3 Mexico
7.3. Eastern Europe Osteogenesis Imperfecta Treatment Market
7.3.1 Key Market Trends, Growth Factors and Opportunities
7.3.2 Top Key Companies
7.3.3 Historic and Forecasted Market Size by Segments
7.3.4 Historic and Forecasted Market Size By By Type
7.3.4.1 Teriparatide
7.3.4.2 Denosumab
7.3.4.3 Others
7.3.5 Historic and Forecasted Market Size By By Route of Administration
7.3.5.1 Subcutaneous
7.3.5.2 Intravenous
7.3.5.3 Oral
7.3.5.4 Others
7.3.6 Historic and Forecast Market Size by Country
7.3.6.1 Russia
7.3.6.2 Bulgaria
7.3.6.3 The Czech Republic
7.3.6.4 Hungary
7.3.6.5 Poland
7.3.6.6 Romania
7.3.6.7 Rest of Eastern Europe
7.4. Western Europe Osteogenesis Imperfecta Treatment Market
7.4.1 Key Market Trends, Growth Factors and Opportunities
7.4.2 Top Key Companies
7.4.3 Historic and Forecasted Market Size by Segments
7.4.4 Historic and Forecasted Market Size By By Type
7.4.4.1 Teriparatide
7.4.4.2 Denosumab
7.4.4.3 Others
7.4.5 Historic and Forecasted Market Size By By Route of Administration
7.4.5.1 Subcutaneous
7.4.5.2 Intravenous
7.4.5.3 Oral
7.4.5.4 Others
7.4.6 Historic and Forecast Market Size by Country
7.4.6.1 Germany
7.4.6.2 UK
7.4.6.3 France
7.4.6.4 The Netherlands
7.4.6.5 Italy
7.4.6.6 Spain
7.4.6.7 Rest of Western Europe
7.5. Asia Pacific Osteogenesis Imperfecta Treatment Market
7.5.1 Key Market Trends, Growth Factors and Opportunities
7.5.2 Top Key Companies
7.5.3 Historic and Forecasted Market Size by Segments
7.5.4 Historic and Forecasted Market Size By By Type
7.5.4.1 Teriparatide
7.5.4.2 Denosumab
7.5.4.3 Others
7.5.5 Historic and Forecasted Market Size By By Route of Administration
7.5.5.1 Subcutaneous
7.5.5.2 Intravenous
7.5.5.3 Oral
7.5.5.4 Others
7.5.6 Historic and Forecast Market Size by Country
7.5.6.1 China
7.5.6.2 India
7.5.6.3 Japan
7.5.6.4 South Korea
7.5.6.5 Malaysia
7.5.6.6 Thailand
7.5.6.7 Vietnam
7.5.6.8 The Philippines
7.5.6.9 Australia
7.5.6.10 New Zealand
7.5.6.11 Rest of APAC
7.6. Middle East & Africa Osteogenesis Imperfecta Treatment Market
7.6.1 Key Market Trends, Growth Factors and Opportunities
7.6.2 Top Key Companies
7.6.3 Historic and Forecasted Market Size by Segments
7.6.4 Historic and Forecasted Market Size By By Type
7.6.4.1 Teriparatide
7.6.4.2 Denosumab
7.6.4.3 Others
7.6.5 Historic and Forecasted Market Size By By Route of Administration
7.6.5.1 Subcutaneous
7.6.5.2 Intravenous
7.6.5.3 Oral
7.6.5.4 Others
7.6.6 Historic and Forecast Market Size by Country
7.6.6.1 Turkiye
7.6.6.2 Bahrain
7.6.6.3 Kuwait
7.6.6.4 Saudi Arabia
7.6.6.5 Qatar
7.6.6.6 UAE
7.6.6.7 Israel
7.6.6.8 South Africa
7.7. South America Osteogenesis Imperfecta Treatment Market
7.7.1 Key Market Trends, Growth Factors and Opportunities
7.7.2 Top Key Companies
7.7.3 Historic and Forecasted Market Size by Segments
7.7.4 Historic and Forecasted Market Size By By Type
7.7.4.1 Teriparatide
7.7.4.2 Denosumab
7.7.4.3 Others
7.7.5 Historic and Forecasted Market Size By By Route of Administration
7.7.5.1 Subcutaneous
7.7.5.2 Intravenous
7.7.5.3 Oral
7.7.5.4 Others
7.7.6 Historic and Forecast Market Size by Country
7.7.6.1 Brazil
7.7.6.2 Argentina
7.7.6.3 Rest of SA
Chapter 8 Analyst Viewpoint and Conclusion
8.1 Recommendations and Concluding Analysis
8.2 Potential Market Strategies
Chapter 9 Research Methodology
9.1 Research Process
9.2 Primary Research
9.3 Secondary Research
|
Global Osteogenesis Imperfecta Treatment Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 722.9 Million |
|
Forecast Period 2024-32 CAGR: |
2.5 % |
Market Size in 2032: |
USD 902.80 Million |
|
Segments Covered: |
By Type |
|
|
|
By Route of Administration |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Frequently Asked Questions :
The forecast period in the Osteogenesis Imperfecta Treatment Market research report is 2024-2032.
Pfizer Inc., Amgen Inc., BioMarin Pharmaceutical Inc., Johnson & Johnson, GlaxoSmithKline plc, and Other Active Players.
The Osteogenesis Imperfecta Treatment Market is segmented into Drugs, Route of Administration and region. By Drug, the market is categorized into Teriparatide, Denosumab, and Others. By Route of Administration, the market is categorized into Subcutaneous, Intravenous, Oral, and Others. By region, it is analyzed across North America (U.S., Canada, Mexico), Eastern Europe (Russia, Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe), Western Europe (Germany, UK, France, The Netherlands, Italy, Spain, Rest of Western Europe), Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New-Zealand, Rest of APAC), Middle East & Africa (Turkiye, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa), South America (Brazil, Argentina, Rest of SA).
The Osteogenesis Imperfecta (OI) Treatment Market includes treatments, medications and therapies employed for easing the conditions of osteogenesis imperfecta, a rare genetic disorder that results in bone fragility, bone fractures and skeletal abnormalities. Management techniques centre on preventing bone loss, preventing bone fractures and promoting the general wellbeing of patients through medication, operations and physiotherapy.
Osteogenesis Imperfecta Treatment Market Size Was Valued at USD 722.9 Million in 2023, and is Projected to Reach USD 902.80 Million by 2032, Growing at a CAGR of 2.5 % From 2024-2032.








